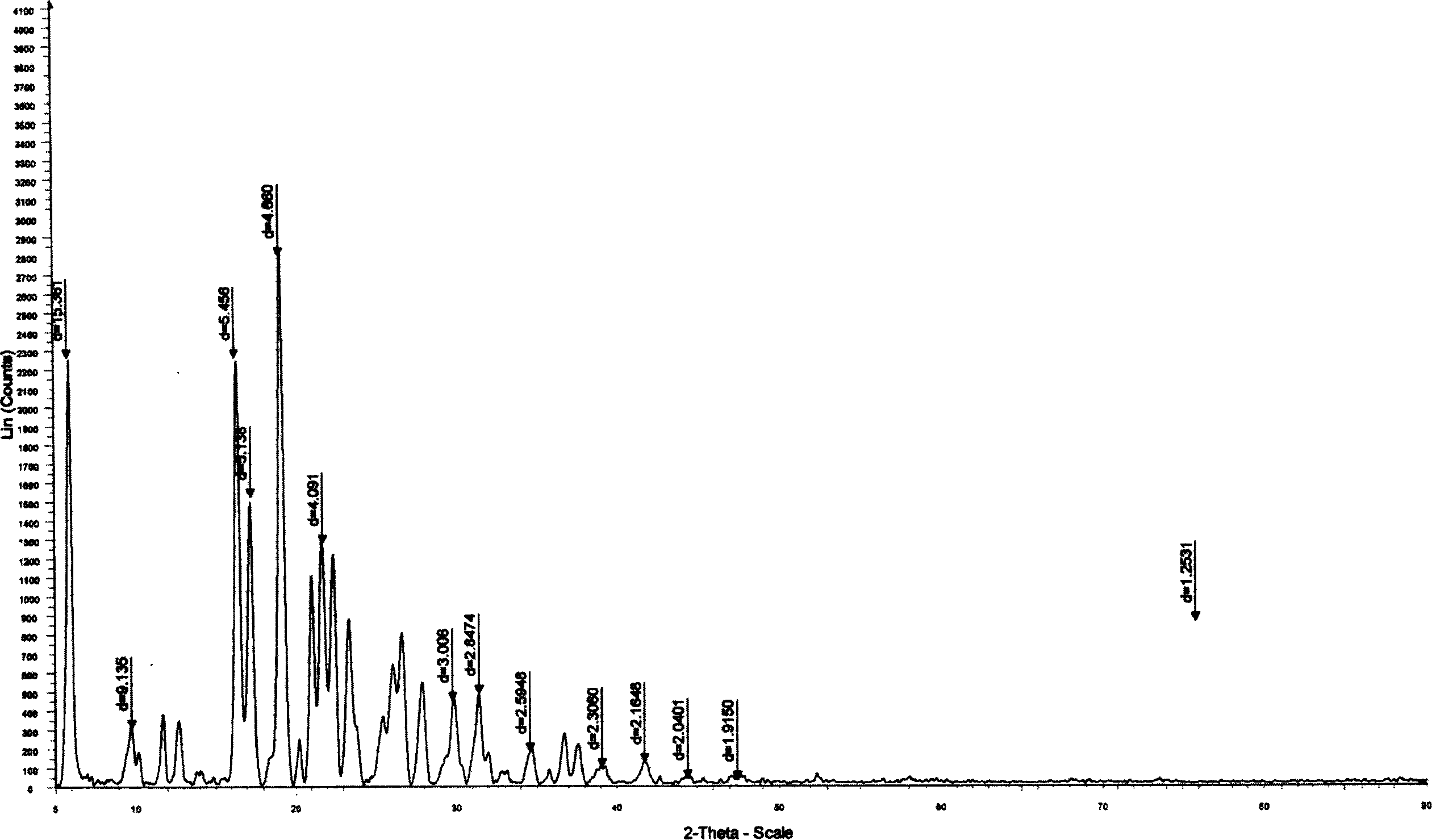
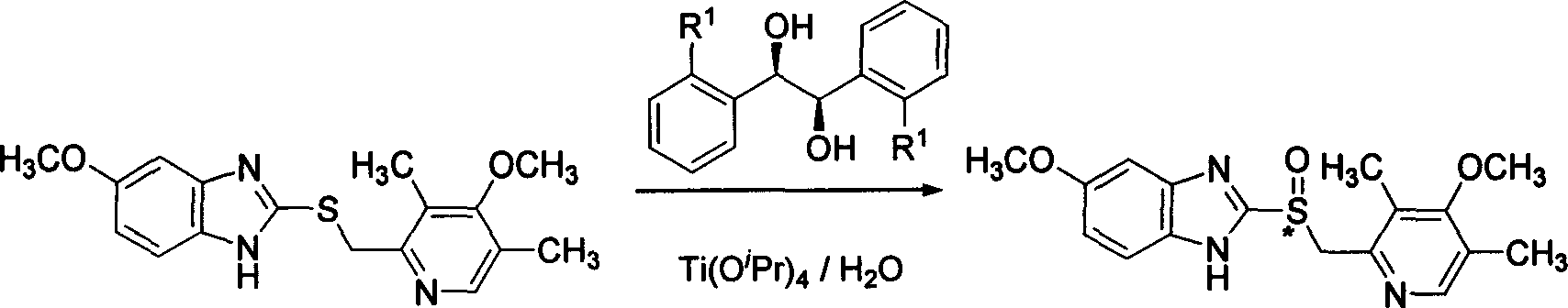
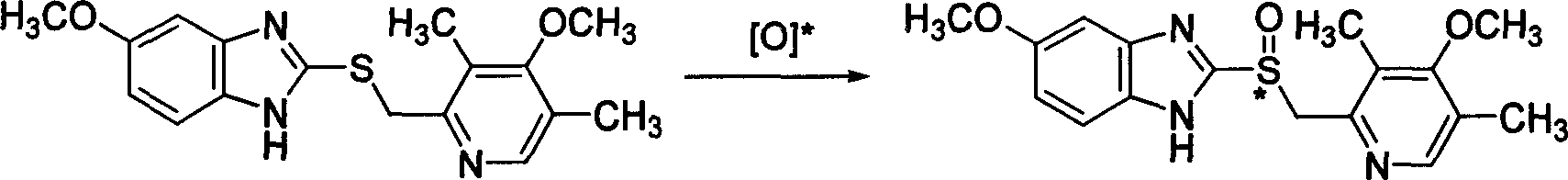Selective prepn process of (S)-Omeprazole with high antimer
A technology of enantioselectivity and omeprazole, which is applied in pharmaceutical formulations, organic active ingredients, medical preparations containing active ingredients, etc., can solve the problem of large usage of chiral ligands, increased cost and operational complexity , waste of omeprazole raw materials and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1 general operation
[0059] The chiral bidentate ligand (0.16mmol) was dissolved in 5mL of organic solvent, and titanium tetraisopropoxide (0.08mmol) was added under stirring, then water (1.6mmol) was added, stirring was continued, and omeprazole sulfide (1.6mmol) was added. mmol), be down to-20 DEG C, then slowly add oxidizing agent (3.2mmol) dropwise, continue reaction 12 hours, aftertreatment, promptly can obtain omeprazole. 1 H NMR (300MHz, CDCl 3 ): 2.20(s, 3H), 2.22(s, 3H), 3.67(s, 3H), 3.83(s, 3H), 4.77(m, 2H), 6.88-6.95(m, 2H), 7.62(m, 1H), 8.20(s, 1H), 12.18(bs, 1H). Chiral HPLC conditions are: chiral Chiralpak The AD analysis column uses ethanol:n-hexane:acetic acid=50:50:5 as the mobile phase, the flow rate is 0.7mL / min, and the ultraviolet monitoring is at 302nm. retention time is t S =10.28,t R =15.39.
Embodiment 2
[0060] The optimization of embodiment 2 reaction conditions
[0061] The experimental operation is the same as in Example 1, and the feeding ratio is as follows: Omeprazole sulfide: (R, R)-1,2-bis(2-bromo-phenyl)-1,2-diol: titanium tetraisopropoxide :water:oxidant molar ratio is 1:0.1:0.05:1:2. The bidentate ligand used in this reaction is (R,R)-1,2-bis(2-bromo-phenyl)-1,2-diol, and the experimental results are shown in Table 1.
[0062] serial number
[0063] 3
[0064] Note: Ee is enantioselectivity. CCl 4 is carbon tetrachloride. CH 2 Cl 2 is dichloromethane. Toluene is toluene. TBHP is tert-butyl hydroperoxide. CHP is cumene hydroperoxide.
Embodiment 3
[0065] The influence of embodiment 3 ligand substituent effect on reaction
[0066] The experimental operation is the same as in Example 1, and the feeding ratio is as follows: Omeprazole sulfide: (R, R)-1,2-bis(2-bromo-phenyl)-1,2-diol derivative: tetraisopropyl The molar ratio of titanium alkoxide: water: oxidizing agent is 1:0.1:0.05:1:2. The solvent used in this reaction is toluene, and the oxidizing agent is tert-butyl hydroperoxide. The experimental results are shown in Table 2.
[0067] Table 2
[0068]
[0069] serial number
[0070] 8
[0071] Note: Ee is enantioselectivity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com