Production of aluminium hydrate from aluminium ash
A technology of aluminum hydroxide and aluminum ash, which is applied in the preparation of alkali metal aluminate/aluminum oxide/aluminum hydroxide, aluminum sulfur compounds, aluminum sulfate, etc., can solve the problems of waste of resources, secondary pollution of the environment, etc., and achieve cost reduction Low cost, avoid secondary pollution, and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
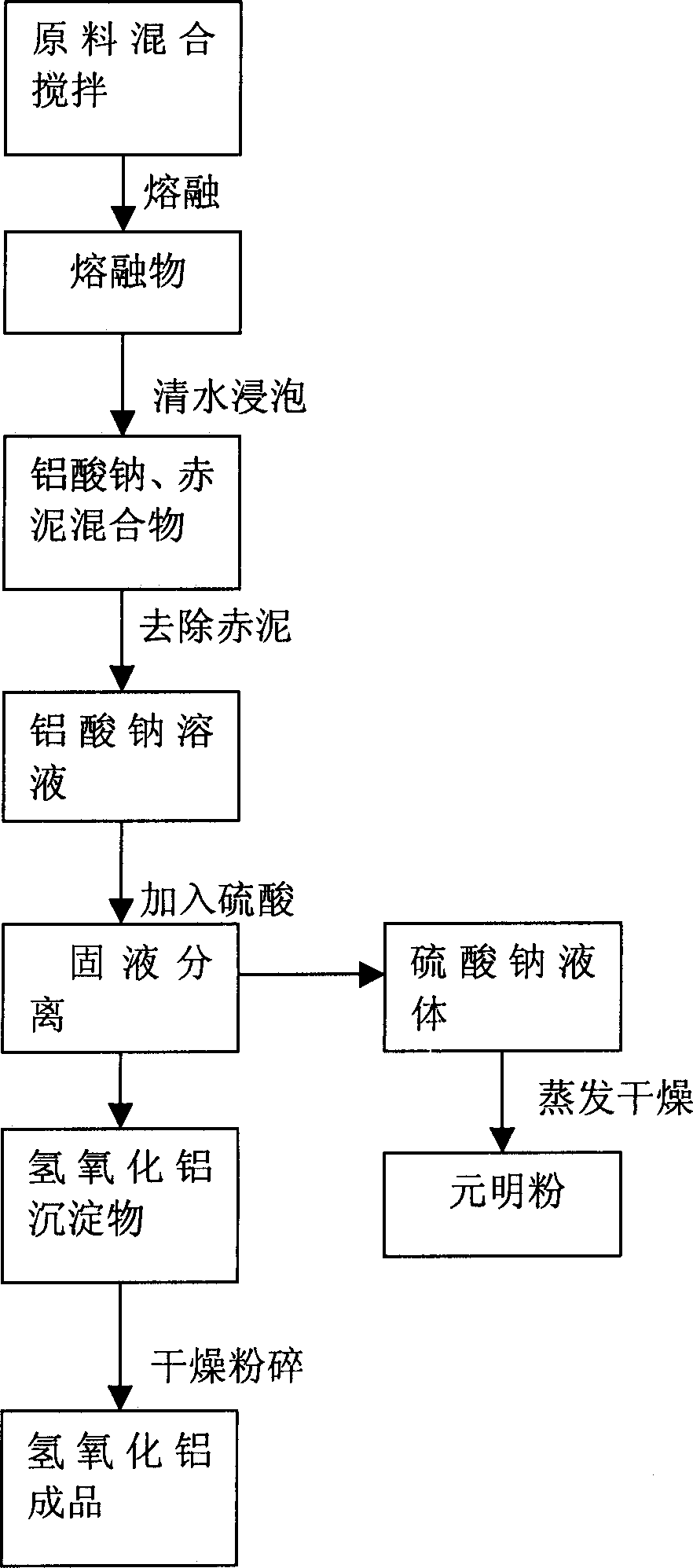
[0016] Embodiment one: a kind of method utilizing aluminum ash to prepare aluminum hydroxide, it comprises the following steps: 1. aluminum ash and solid sodium carbonate are mixed by weight ratio 100:100 and stirred in the mixer; 2. solid mixture is put into Melt in a high-temperature furnace at 1100°C for 3 hours; ③Put the molten material in a leaching tank and soak it in clean water for 12 hours, and remove the non-polluting red mud sediment to leave a sodium aluminate solution; ④Molar ratio of aluminum Sodium acid: sulfuric acid is 2: 1. Add sulfuric acid to the sodium aluminate solution in the reaction kettle; ⑤ Use a filter to separate the solid and liquid from the mixed solution to obtain aluminum hydroxide precipitate and sodium sulfate solution; ⑥ Add aluminum hydroxide After the precipitate is dried and pulverized at 60°C, the finished aluminum hydroxide product can be obtained, and the sodium sulfate solution can be evaporated and dried to obtain the chemical raw mat...
Embodiment 2
[0017] Embodiment two: a kind of method that utilizes aluminum ash to prepare aluminum hydroxide, it comprises the following steps: 1. aluminum ash and solid sodium carbonate are mixed by weight ratio 100:80 and stirred in the blender; 2. solid mixture is put into Melt in a high-temperature furnace at 900°C for 5 hours; ③Put the molten material in a leaching tank and soak it in clean water for 15 hours, and remove the non-polluting red mud sediment to leave a sodium aluminate solution; ④Molar ratio of aluminum Sodium acid: sulfuric acid is 2: 0.95. Add sulfuric acid to the sodium aluminate solution in the reaction kettle; ⑤ Use a filter to separate the solid and liquid from the mixed solution to obtain aluminum hydroxide precipitate and sodium sulfate solution; ⑥ Add aluminum hydroxide After the precipitate is dried and pulverized at 80°C, the finished product of aluminum hydroxide can be obtained, and the sodium sulfate solution can be evaporated and dried to obtain the chemic...
Embodiment 3
[0018] Embodiment three: a kind of method that utilizes aluminum ash to prepare aluminum hydroxide, it comprises the following steps: 1. aluminum ash and solid sodium carbonate are mixed by weight ratio 100: 120 and stirred in the mixer; 2. solid mixture is put into Melt in a high-temperature furnace at 1500°C for 1 hour; ③Put the molten material in a leaching tank and soak it in clean water for 10 hours, remove the non-polluting red mud sediment and leave a sodium aluminate solution; ④Molar ratio of aluminum Sodium acid: sulfuric acid is 2: 1.05. Add sulfuric acid to the sodium aluminate solution in the reaction kettle; ⑤ Use a filter to separate the solid and liquid from the mixed solution to obtain aluminum hydroxide precipitate and sodium sulfate solution; ⑥ Add aluminum hydroxide After the precipitate is dried and pulverized at 40°C, the finished product of aluminum hydroxide can be obtained, and after the sodium sulfate solution is evaporated and dried, the chemical raw m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com