Solubilization carrier for drug, its preparation method and application
A drug and carrier technology, applied in the direction of pharmaceutical formulations, medical preparations of non-active ingredients, freeze-dried delivery, etc., can solve problems such as hemolysis, turbidity, dilution instability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] Preparation of 6-O-carboxymethyl-2-N-octyl chitosan (connecting hydrophilic group first and then hydrophobic group)
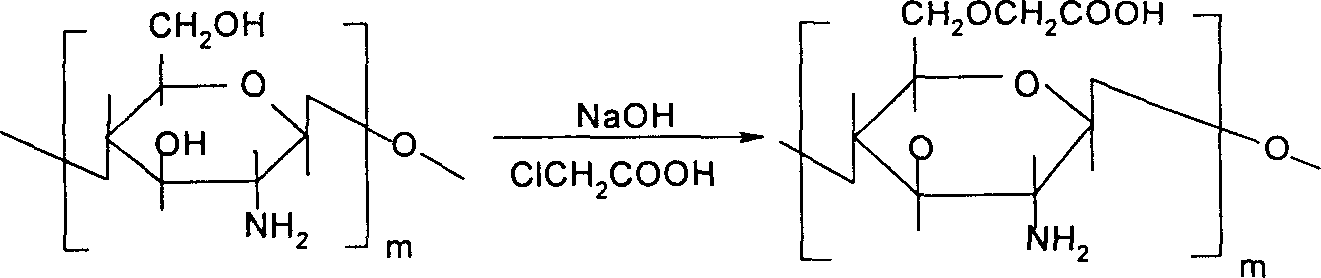
[0121] 1. Preparation of 6-O-carboxymethyl chitosan
[0122] Suspend 1g of chitosan in isopropanol, add 5g of NaOH, stir at 30°C for 12h, add 6g of chloroacetic acid in isopropanol, react at 35°C for 5h, pour off the supernatant, add a certain amount of water, and use Adjust the pH to 7 with NaOH aqueous solution, precipitate with methanol, filter, wash with 90% ethanol and dry in vacuum to obtain highly substituted 6-O-carboxymethyl chitosan as light yellow or white powder.
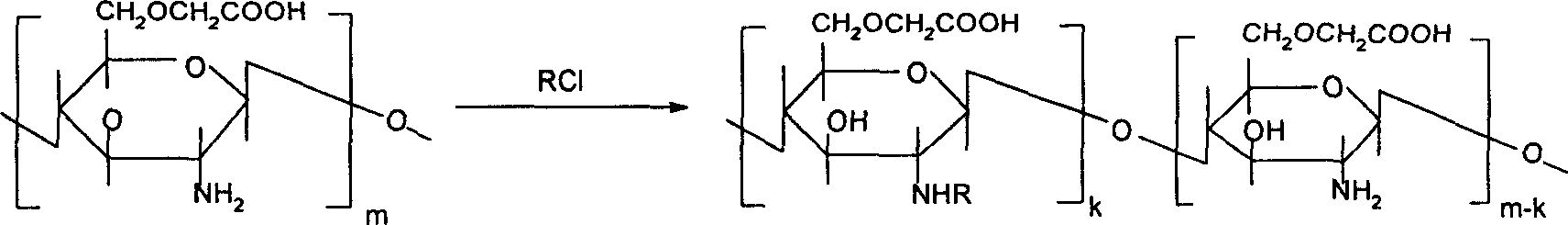
[0123] 2. Preparation of 6-O-carboxymethyl-2-N-octyl chitosan
[0124] A reacts with haloalkane
[0125] Disperse 1g of 6-O-carboxymethyl chitosan evenly in isopropanol, add 2g of NaOH, alkalinize at 35°C, add n-octyl chloride under stirring, react at 30°C for 8h, adjust pH to 7, add acetone to precipitate, filter, filter The cake was washed with ether, and the sample was vacuum...
Embodiment 2
[0131] Preparation of 6-O-carboxymethyl-2-N-octyl chitosan (first connect hydrophobic group and then connect hydrophilic group)
[0132] 1. Preparation of 2-N-octyl chitosan
[0133] The method is the same as in Example 1, 2, replacing 6-O-carboxymethyl chitosan with chitosan.
[0134] 2. Preparation of 6-O-carboxymethyl-2-N-octyl chitosan
[0135] Method is with embodiment 1 middle 1, replaces chitosan with 2-N-octyl chitosan
[0136] Elemental analysis showed that the deacetylation degree was 91.5%, the carboxymethyl substitution degree was 95%, and the octyl substitution degree was 48%.
Embodiment 3
[0138] Preparation of 6-O-carboxymethyl-2-N-decyl chitosan
[0139] Replace n-octyl chloride and n-octyl aldehyde in embodiments 1 and 2 with n-decyl chloride and n-decyl aldehyde, and the preparation method is the same as in embodiments 1 and 2.
[0140] Elemental analysis showed that the deacetylation degree was 91.5%, the carboxymethyl substitution degree was 98%, and the decyl substitution degree was 40%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com