Continuous production method for tetrachloro terephthalonitrile
A technology of tetrachloroterephthalonitrile and terephthalonitrile, applied in chemical instruments and methods, organic chemistry, carboxylic acid nitrile preparation, etc., can solve the problems of temperature sensitivity, no industrial production, poor product quality, etc. Achieve the effect of prolonging service life and improving production capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
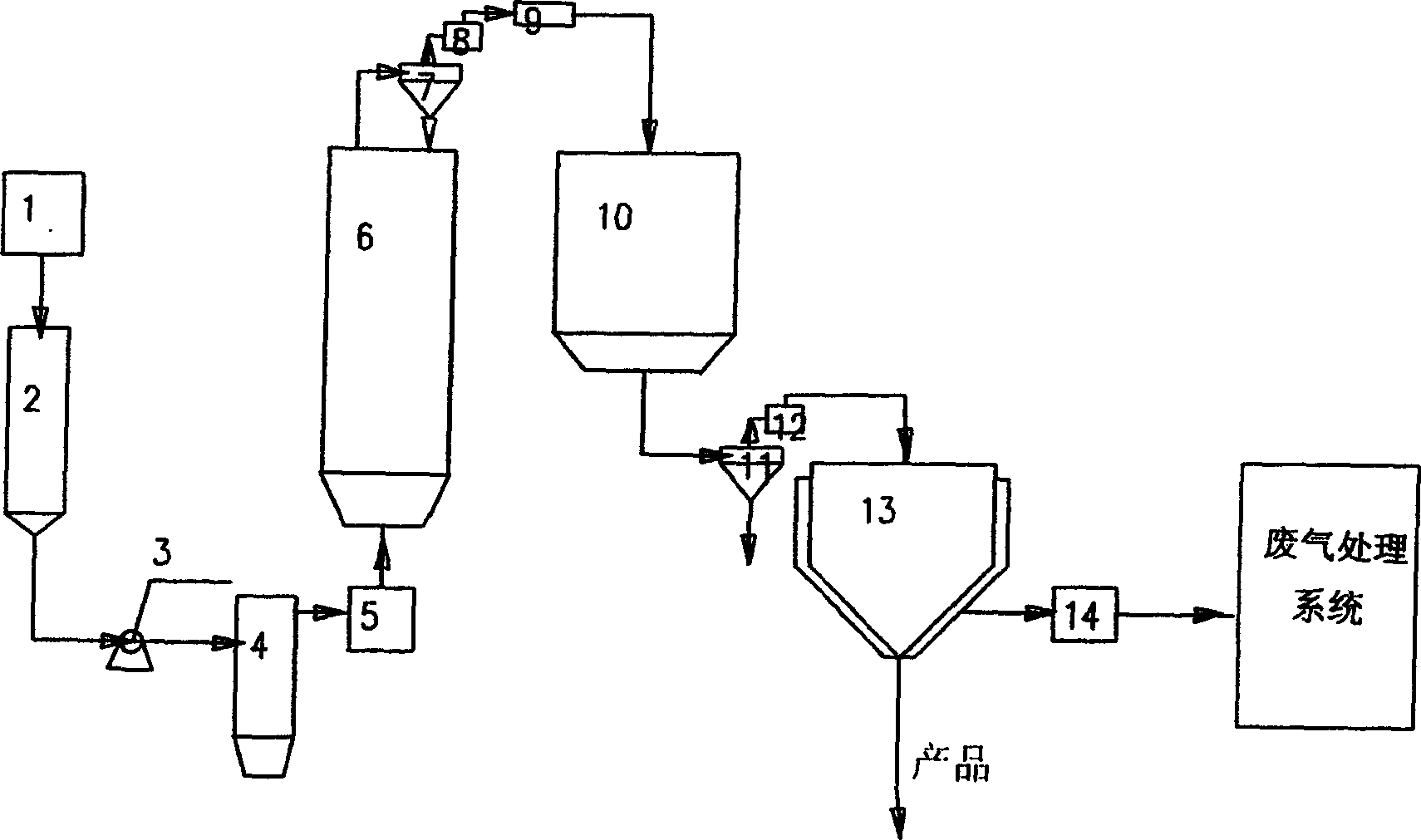
[0035] Embodiment 1: Both fluidized bed and fixed bed do not add modification catalyst
[0036] Add 800kg of ordinary coconut shell activated carbon catalyst of 10-60 mesh into the fluidized bed 6, and add 1000kg of ordinary coconut shell activated carbon catalyst mixed with four different particle sizes into the fixed bed, among which 100kg of particle size is 6-12 mesh, and the particle size is 12-16 mesh. 400kg, particle size 16-24 mesh 400kg, particle size 24-32 mesh 100kg. Heat up and dehydrate for about 4-5 hours. After the dehydration is completed, pass 1 kmol / h of chlorine gas to activate until the temperature of each point of the two reactors is stable, and no HCl can be detected in the tail gas. Depending on the completion of the activation, the activation time is generally 5-6 hours, and the temperature is controlled at 180-220 ° C. within range. Next, molten terephthalonitrile (purity ≥ 98.5%) is sent to a wiped-film evaporator at a speed of 0.5 kmol / h for vapori...
Embodiment 2
[0039] Embodiment 2: (only add modified catalyst in the fluidized bed)
[0040] Embodiment 2 basic process is as described in example 1, and the added catalyst of fluidized bed is modified catalyst, and it is that the FeCl of 15% A and 0.01% have been added in the 10-60 purpose common fruit shell active carbon catalyst of 800kg 3 , A is the chloride of the first group of alkali metals or the second group of alkaline earth metals, especially one of the chlorides of potassium, calcium and barium or their mixture; while the fixed bed is still commonly used by four different The shell activated carbon catalyst with mixed particle size includes 100 kg of particle size 6-12 mesh, 200 kg of particle size 12-16 mesh, 500 kg of particle size 16-24 mesh, and 200 kg of particle size 24-32 mesh. The fluidized bed reaction temperature is 240-280°C, and the fixed bed reaction temperature is controlled at 250-300°C. The obtained product (0.492kmol / h) contains tetrachloroterephthalonitrile ≥...
Embodiment 3
[0041] Embodiment 3: (add modified catalyst only in fixed bed)
[0042] The basic process of embodiment 3 is as described in Example 1. What the fluidized bed uses is still the common coconut shell activated carbon catalyst of 800kg10~60 mesh; and the catalyst added by the fixed bed is a modified catalyst, which is 6-12 mesh and 16- 20% of A and 0.01% of FeCl are added to the common fruit shell activated carbon catalyst mixed with two particle sizes of 24 mesh in a ratio of 1:4 3 . A is a hydroxide, an inorganic salt, an organic acid salt or a mixture thereof of an alkali metal of the first group or an alkaline earth metal of the second group. The fluidized bed reaction temperature is 250-300°C, and the fixed bed reaction temperature is controlled at 250-300°C. The obtained product (0.490 kmol / h) contains tetrachloroterephthalonitrile ≥ 98.0%, HCB ≤ 100 ppm. The service life of the catalyst is 25 days.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com