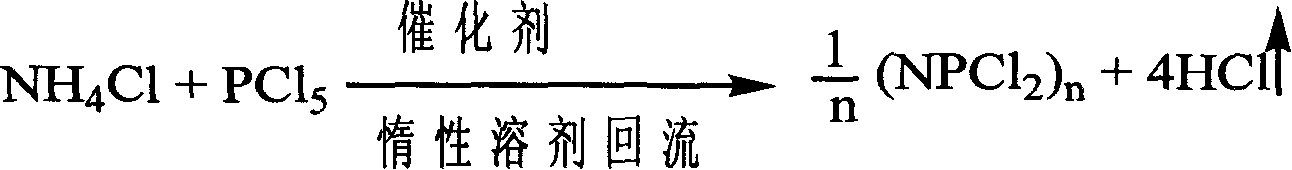
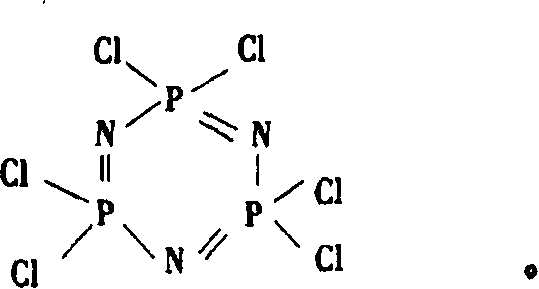
Catalytic synthesizing method of hexa chloro cyclotripolyphosphazene
A technology for hexachlorocyclotripolyphosphazene and a synthesis method, which is applied in chemical instruments and methods, compounds of elements of Group 5/15 of the periodic table, catalysts for physical/chemical processes, etc., can solve the problem of hexachlorocyclotripolyphosphazene High production cost, limited application scope, complicated operation, etc., to achieve the effect of low cost, shortened response time and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: 0.1200mol ammonium chloride, 0.0017mol magnesium chloride and 0.0050mol pyridine are added in the reaction kettle, add 110ml chlorobenzene heating and dissolving, reflux; Under reflux state, add 0.1000mol phosphorus pentachloride 5~10 times, Complete the addition within 2 hours; then continue to reflux for 3 hours, the system turns light yellow-green; stop heating, cool to room temperature, filter out unreacted ammonium chloride, and then distill chlorobenzene; add 56ml of petroleum ether to the residue and heat under reflux for extraction 0.5~1h, cooling, filtering, distilling petroleum ether to obtain orange or white crystals, recrystallization or sublimation with n-heptane to obtain pure white crystal product hexachlorocyclotrimeric phosphazene, the yield is 88%, and the melting point is 111 ~113°C, 31 P(DMSO): 20.5ppm.
Embodiment 2
[0030] Example 2: Add 0.1200mol of ammonium chloride, 0.0017mol of cobalt chloride and 0.0050mol of pyridine into the reactor, add 110ml of chlorobenzene to heat and dissolve, and reflux; under reflux, add 0.1000mol of pentachloride in 5 to 10 times Add phosphorus within 2 hours; then continue to reflux for 5 hours, the system turns light yellow-green; stop heating, cool to room temperature, filter out unreacted ammonium chloride, and then distill chlorobenzene; add 56ml of petroleum ether to the residue and heat Reflux extraction for 0.5-1h, cooling, filtering, and distilling petroleum ether to obtain orange or white crystals, recrystallization or sublimation with n-heptane to obtain pure white crystal product hexachlorocyclotrimeric phosphazene, the yield rate is 83%, the melting point 111~113℃, 31 P(DMSO): 20.5ppm.
Embodiment 3
[0031] Example 3: Add 0.1200mol ammonium chloride, 0.0017mol zinc chloride and 0.0050mol pyridine into the reaction kettle, add 110ml chlorobenzene to heat and dissolve, and reflux; in the reflux state, add 0.1000mol pentachloride in 5 to 10 times Add phosphorus within 2 hours; then continue to reflux for 5 hours, the system turns light yellow-green; stop heating, cool to room temperature, filter out unreacted ammonium chloride, then distill chlorobenzene, add 56ml of petroleum ether to the residue and heat Reflux extraction for 0.5-1h, cooling, filtering, and distilling petroleum ether to obtain orange or white crystals, recrystallization or sublimation with n-heptane to obtain pure white crystal product hexachlorocyclotrimeric phosphazene, the yield rate is 83%, the melting point 111~113℃, 31 P(DMSO): 20.5ppm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com