Production method of recombination human interferon gamma
A technology of interferon and gamma protein, applied in the field of genetic engineering, can solve the problems of low interferon activity, difficult protein renaturation, complex process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1 Construction of expression plasmids and acquisition of high-expression engineering strains
[0063] According to the natural amino acid sequence of human interferon gamma, according to the codon preference, under the condition of not changing the amino acid sequence, the target gene sequence encoding the mature peptide of human interferon gamma protein was synthesized, and the gene sequence was cloned into pUC19 and sequenced verify.
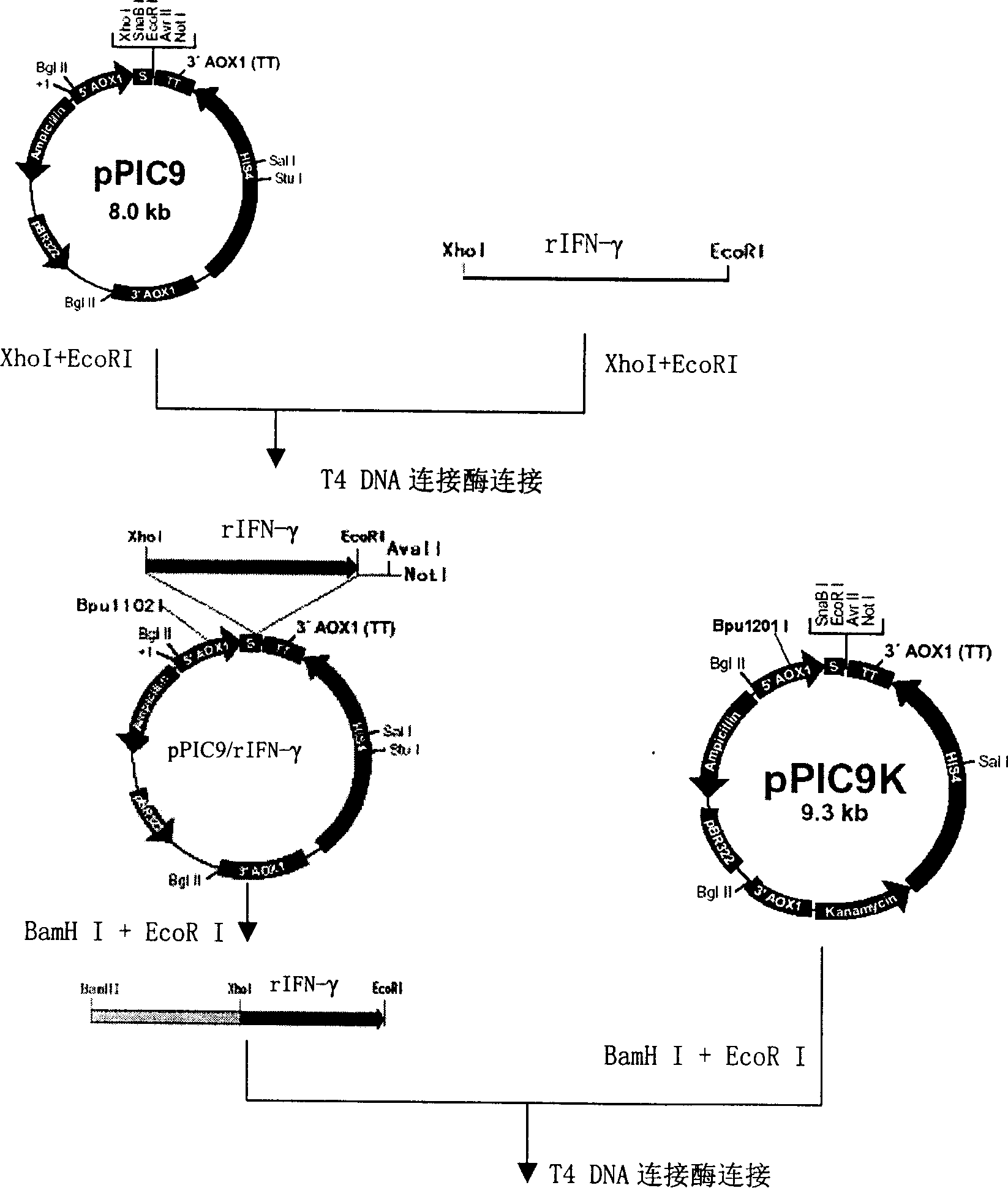
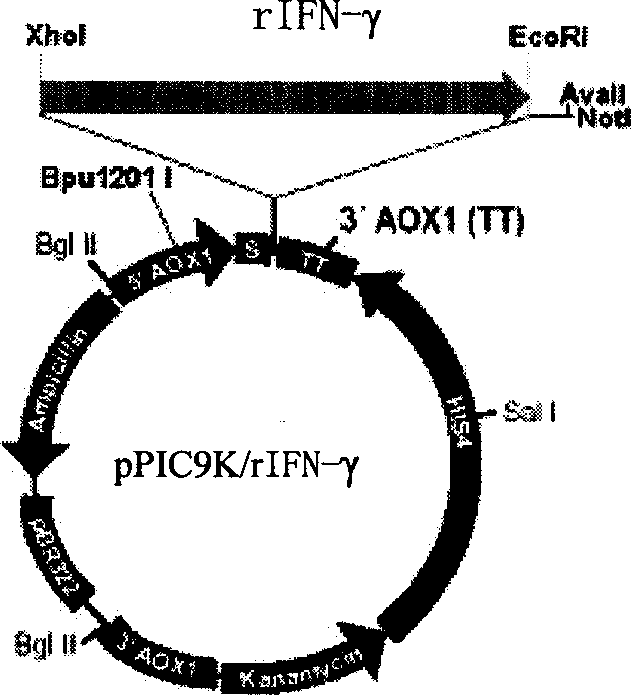
[0064] For expression vector construction methods, see figure 1 . The target human interferon gamma gene was amplified by PCR, and double-digested with XhoI+EcoRI (MBI, 2*Tango TM , 37°C) PCR product and pPIC9 plasmid (purchased from Invitrogen). The two fragments were ligated with T4 DNA ligase, and the ligated product was transformed into Escherichia coli DH5α (purchased from Promega Company), clones were selected on an LB plate containing ampicillin, a small amount of plasmid was prepared, and screening was performed by do...
Embodiment 2
[0066] Example 2 Effect of different pH on expression level
[0067] Take a single clone, inoculate it into the BMGY primary seed solution, and cultivate it for 17-20hr; secondly inoculate it in a 500ml Erlenmeyer flask with 50ml medium at a ratio of 1:10 (each condition has an auxiliary tube), and cultivate it for about 24hr, 1 % methanol induction, the pH of the induction stage needs to be adjusted (in the BMMY medium, the pH is directly adjusted with a phosphate buffer system). Sampling was taken at 24 hours after induction, OD and pH were measured at the same time, and 1% methanol was added to continue induction. A total of 48hr induction. SDS-PAGE of samples before induction and induction at different times.
[0068] The experimental results showed that the optimum pH for expressing IFN-γ at the shake flask level was between 5 and 9, and the optimum pH was 5-7.
Embodiment 3
[0069] Example 3 The effect of adding different protein protectants on the expression level during the induction phase
[0070] Take a single clone, inoculate it into the BMGY primary seed liquid, and cultivate it for 17-20hr; secondly inoculate it in a 1L Erlenmeyer flask of 250ml BMGY at a ratio of 1:10, culture it for about 4~8hr, put it into the tank for fermentation, pH 5.0, temperature 20°C, D0 > 35%, after the dissolved oxygen rises, add 50% glycerin at 10~15 rpm. After the glycerin is exhausted and the dissolved oxygen rises again, use 100% methanol to start induction at speed 1, and gradually increase the speed. In the induction phase, 300ml of CA, Peptone and Tryptone with a concentration of 10% were added to the tank (5L fermenter, 3L fermentation supernatant), and the induction was over for 48hrs. Samples were tested for SDS-PAGE and protein content to determine the best protein protectant for the induction phase.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com