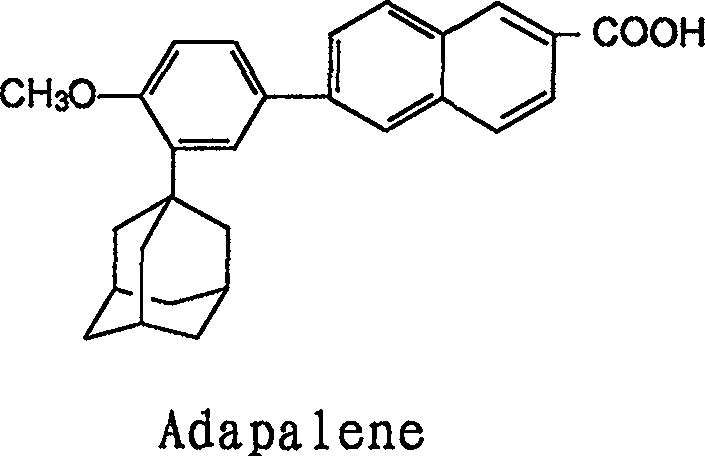
Method for preparing Adapalene
An equation and reaction technology, applied in the preparation of carboxylate/lactone, organic chemistry, etc., can solve the problems of three wastes, high cost, difficult industrial production, etc., and achieve the effects of reducing three wastes, easy operation and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
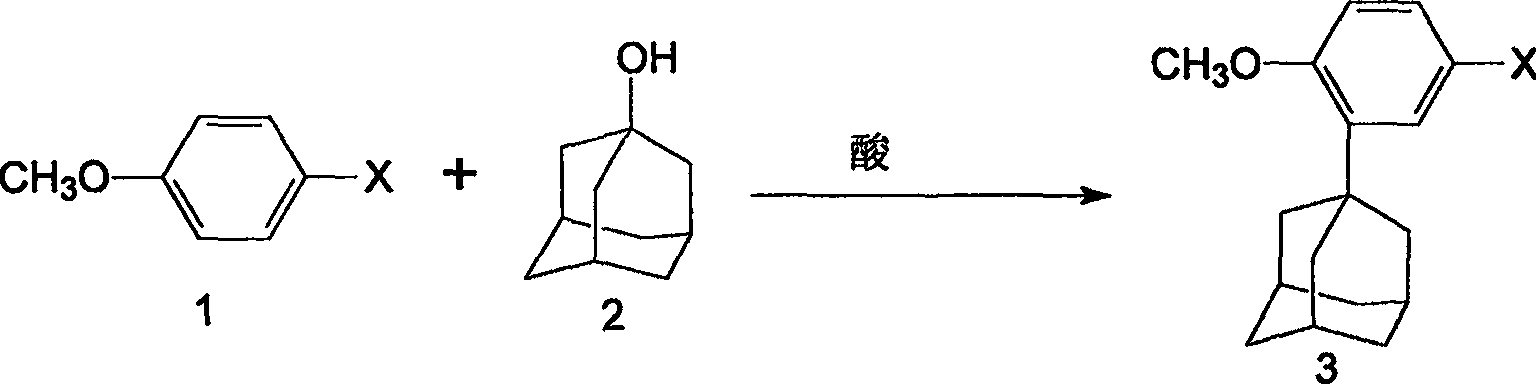
[0013] Preparation of 2-(1-adamantyl)-4-bromoanisole
[0014] Add 14.02g of p-bromoanisole, 11.4g of 1-adamantol and 250ml of dichloromethane into a 500ml reactor, stir and dissolve, add 3.75ml of concentrated sulfuric acid dropwise at room temperature, and react at room temperature for 8 hours. The reaction mixture was poured into 250ml of water, allowed to stand to separate layers, and the dichloromethane layer was washed three times with 20ml of saturated sodium bicarbonate solution, and then washed with water until neutral. The lower organic phase was separated and concentrated under reduced pressure to obtain a crude product. The crude product was dissolved with an appropriate amount of petroleum ether, filtered through silica gel, washed with an appropriate amount of petroleum ether, and the eluent was vacuum-precipitated to obtain 18.3 g of a white solid with a yield of 76.1% and a melting point of 136-137°C.
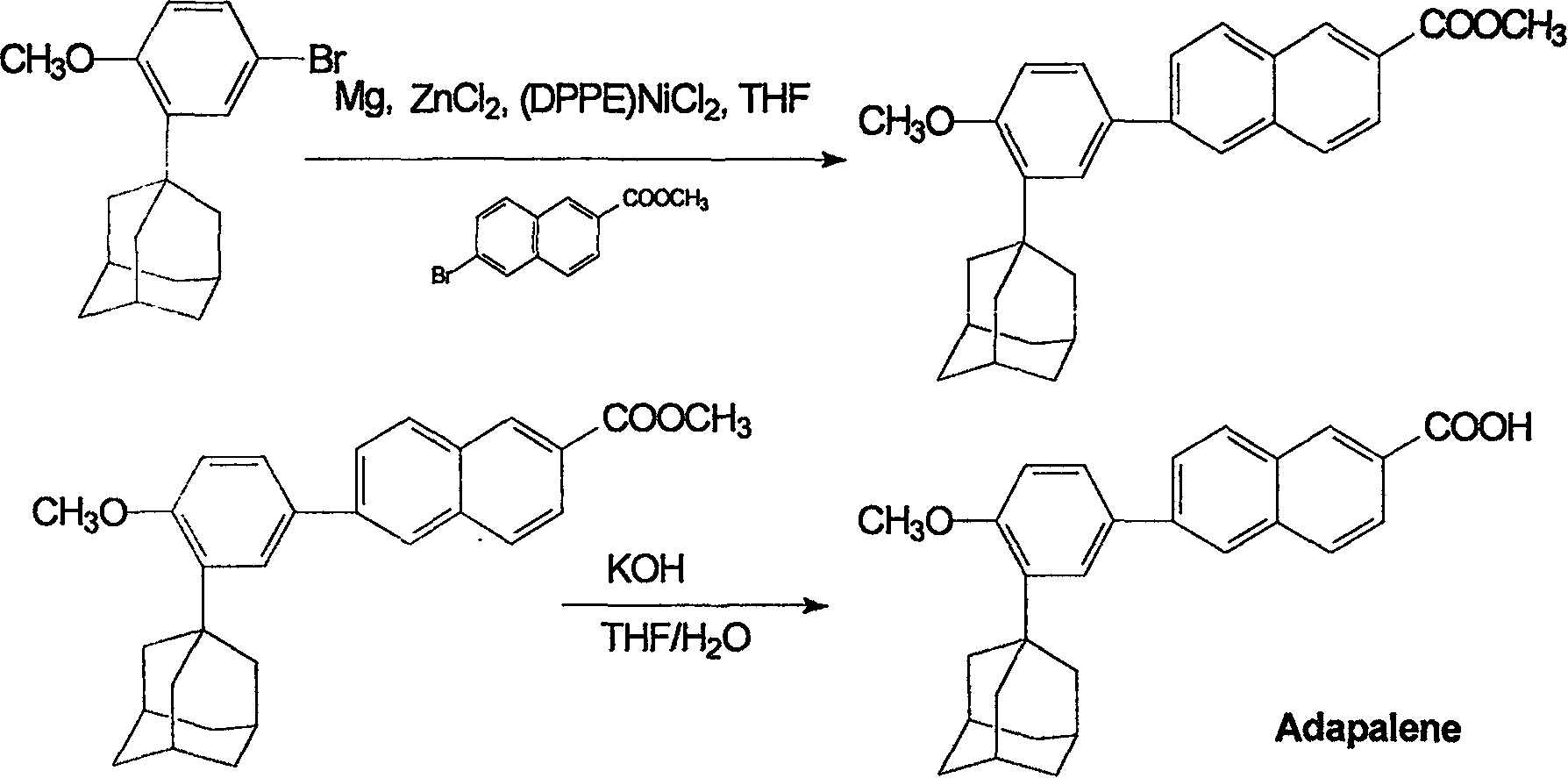
[0015] Preparation of methyl 6-[3-(1-adamantyl)-4-methoxyph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com