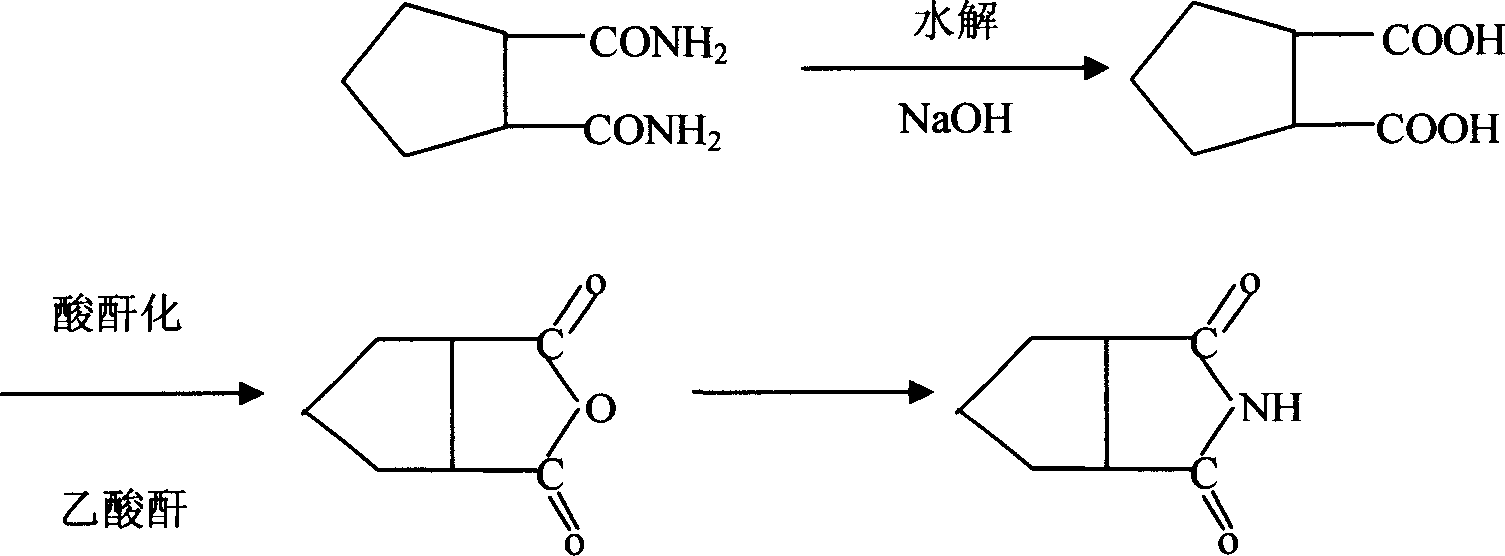
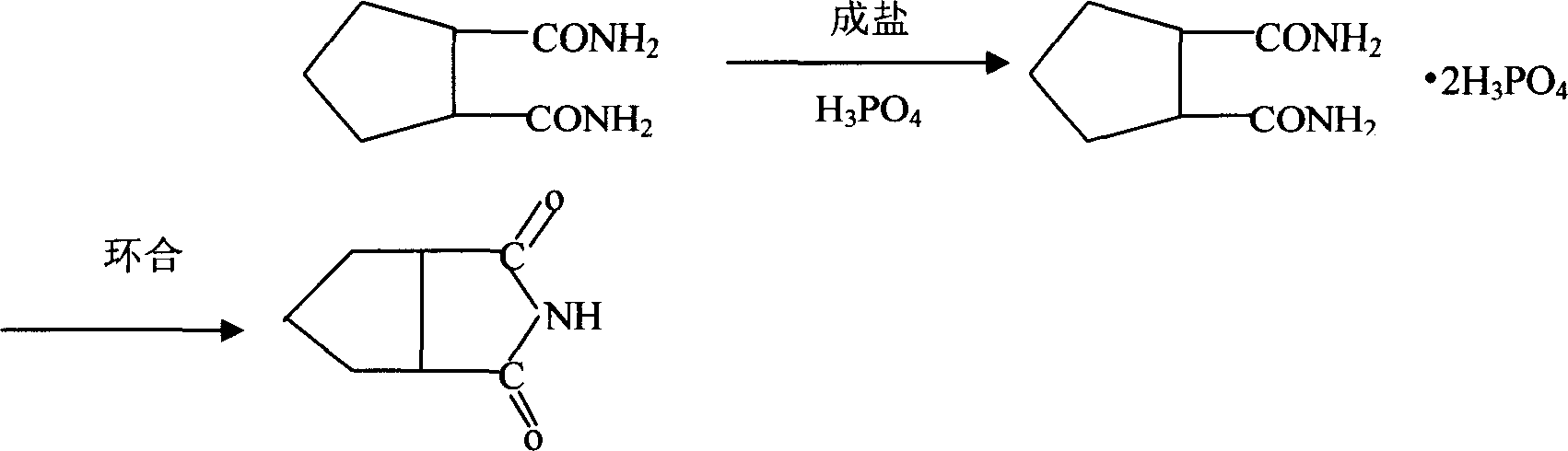
Cyclopentane1,2-diimide preparation method
A technology of diimide and cyclopentane, which is applied in the field of cyclopentane 1, can solve the problems of carbonization, high raw material cost, and low total yield, and achieve the effects of reducing time and cost, increasing yield, and reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] To form a salt, put 500g of 95% ethanol and 100g of cyclopentane 1,2-dicarboxamide into a 1000ml reaction bottle, heat it to 40-55°C to dissolve, add 147g of phosphoric acid dropwise after the dissolution is complete, and precipitate the material into a salt, drop After adding, keep warm for 30 minutes at 40-55°C, then cool with cold water, then cool with ice-salt water to an internal temperature of about 10°C, keep warm for 30 minutes, finish the heat preservation, spin dry by centrifuge, dry in a drying room, and dry to obtain About 225g of amine salt, the consumption of ethanol is 68g.
[0018] For cyclization, put 200g of the amine salt obtained above into a 500ml reaction bottle, and heat it with oil to dissolve the cyclization, heat to an internal temperature of 220-250°C and keep it warm for 2 hours to complete the cyclization reaction. Put the ammonium phosphate in the lower layer, the upper layer is cyclopentane 1,2-imide, and then after refining with toluene, ...
Embodiment 2
[0022] Get 100g of cyclopentane 1,2-dicarboxamide and 128g of 98% sulfuric acid, adopt the method as described in Example 1 to obtain 225g of amine salt, and the yield of amine salt is 100%. Then, the obtained amine salt was cyclized at 260° C. to obtain 66.82 g of cyclopentane 1,2-diimide with a total yield of 74.99%.
Embodiment 3
[0024] Get 100g of cyclopentane 1,2-dicarboxamide and 131g of 36% hydrochloric acid, adopt the method as described in Example 1 to obtain 136g of amine salt, and the yield of amine salt is 92.5%. Then, the obtained amine salt was cyclized at 225° C. to obtain 56.8 g of cyclopentane 1,2-diimide with a total yield of 63.75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com