Beta-hydroxy propyleneimine vanadium olefinic polymerization catalyst and its preparation method and uses
A technology of hydroxypropene imine vanadium olefin and polymerization catalyst is applied in the field of β-hydroxypropene imine vanadium olefin polymerization catalyst and preparation, and can solve the problems of low catalytic activity, poor high temperature resistance, easy deactivation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
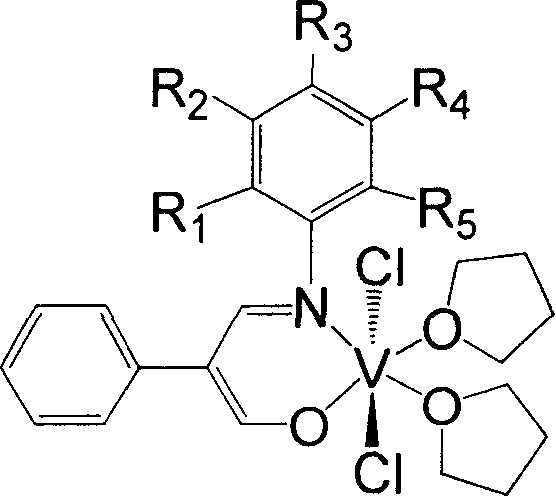
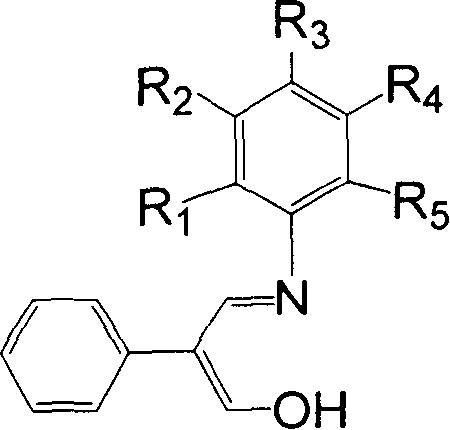
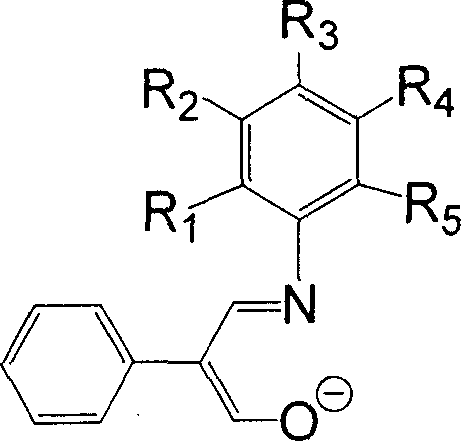
[0032] 11.85 g of 2-phenyl-3-hydroxy-acrolein (equivalent to 80 mmol), 3.73 g of aniline (equivalent to 40 mmol), 30 mL of methanol, and 2 mL of formic acid were added to a dry reactor, and heated to reflux for 24 hours. The solvent methanol was distilled off with a rotary evaporator, and petroleum ether containing 1% ethyl acetate was used as an eluent, and the residue was subjected to column chromatography to obtain 4.55 g of yellow solid Schiff's base, with a yield of 51%. 1H NMR (300MHz, DMSO): δ7.01-7.51 (m, 10H, Ar-H) 7.89-7.94 (d, 1H, CH=N) 9.30-9.35 (d, 1H, C=CHOH) 12.02-12.06 ( b, 1H, OH). According to mass spectrometry, the molecular ion peak m / e is 223. Elemental analysis measured value: C, 80.54%; H, 5.93%; N, 6.16%; Theoretical value (C 15 h 13 NO): C, 80.69%; H, 5.87%; N, 6.27%.
[0033] Under a nitrogen atmosphere, add 0.45 g of the above-obtained Schiffer’s base (equivalent to 2.0 mmol) and 20 mL of anhydrous tetrahydrofuran into a dry reactor, stir at room...
Embodiment 2
[0035] Use 2-phenyl-3-hydroxy-acrolein 5.93g equivalent to 40mmol, use 2-fluoroaniline 2.22g equivalent to 20mmol to replace the aniline, methanol 15mL, and formic acid 1mL in Example 1, heat and reflux for 36h, and the experimental operation is the same In Example 1, 2.36 g of yellow solid Schiff's base was obtained, with a yield of 49%. 1 H NMR (300MHz, CDCl 3 ): δ7.16-7.66 (m, 10H, Ar-H+CH=N) 9.76-9.77 (d, 1H, C=CHOH) 12.13-12.16 (b, 1H, OH). According to mass spectrometry, the molecular ion peak m / e is 241. Elemental analysis measured value: C, 74.51%; H, 5.13%; N, 5.92%; Theoretical value (C 15 h 12 FNO): C, 74.67%; H, 5.01%; N, 5.81%.
[0036] Using 0.48 g of the Schiffs base prepared in Example 2 is equivalent to 2 mmol to replace the Schiffs base obtained in Example 1, and the experimental operation was the same as in Example 1 to obtain 0.45 g of the brown complex with a yield of 45%. According to mass spectrometry, the molecular ion peak m / e is 505. Elemental a...
Embodiment 3
[0038] Replace the aniline in Example 1 with 8.89g of 2-phenyl-3-hydroxy-propenal equivalent to 60mmol, 4.83g of 3-trifluoromethylaniline equivalent to 30mmol, methanol 20mL, formic acid 1.5mL, heat and reflux for 48h , the experimental operation was the same as in Example 1, and 4.72 g of yellow solid Schiff's base was obtained, with a yield of 54%. According to mass spectrometry, the molecular ion peak m / e is 291. 1 H NMR (300MHz, CDCl 3 ): δ7.29-7.60 (m, 9H, Ar-H) 7.60-7.65 (m, 1H, CH=N) 9.73-9.74 (d, 1H, C=CHOH) 12.15-12.19 (b, 1H, OH) . Elemental analysis measured value: C, 65.83%; H, 4.09%; N, 4.73%; Theoretical value (C 16 h 12 f 3 NO): C, 65.98%; H, 4.15%; N, 4.81%.
[0039] Using 0.58 g of Schiffer's base prepared in Example 3, equivalent to 2 mmol, to replace the Schiffer's base obtained in Example 1, the experimental operation was the same as in Example 1, and 0.48 g of the brown complex was obtained, with a yield of 43%. According to mass spectrometry, the m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com