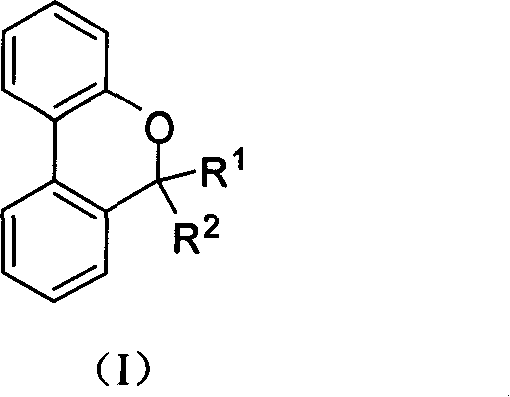
Dibenzanthracene, dinaphthopyran and dibenzanthracene, dinaphtho spiropyran analog compound and its preparation
A technology of benzospiropyran and naphthospiropyran, which is applied in the field of dibenzo, dinaphthospiropyran and dibenzo, dinaphthospiropyran compounds and their preparation, can solve complex steps, The overall yield is low, the raw materials are not easy to obtain, etc., to achieve the effect of simple steps, easy to obtain raw materials, and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Synthesis of Compound 1-19:
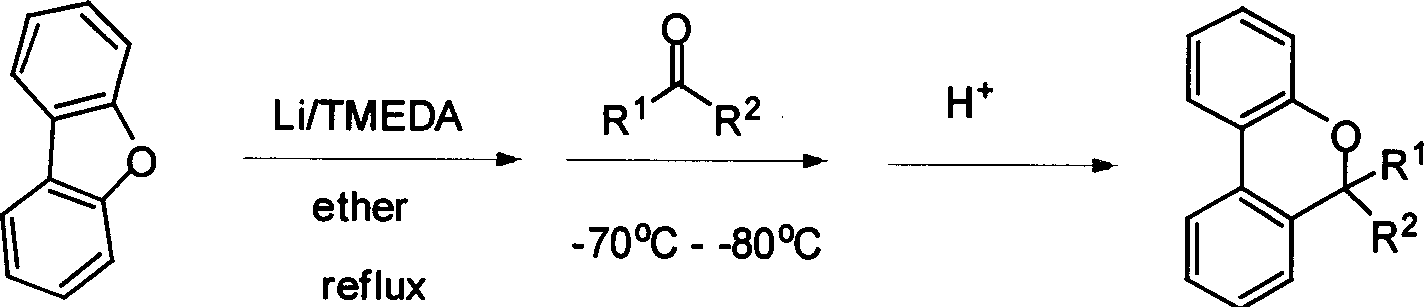
[0030] In a 100mL three-neck flask equipped with a magnet, add 0.153g (22mmol) lithium, 1.680g (10mmol) dibenzofuran, 3.30mL (22mmol) tetramethylethylenediamine and 50mL dry diethyl ether, and heat to reflux for 24 hours . 0.59 mL (8 mmol) of acetone was added at -78°C, the reaction was gradually raised to room temperature, and stirred overnight. Then slowly add 3N dilute hydrochloric acid for hydrolysis, separate the liquid, add 20mL concentrated hydrochloric acid to the organic phase, stir at room temperature for 2 hours, separate the liquid, and wash the aqueous phase with CH 2 Cl 2 Extract (20mL×4), combine the organic phases, wash with water until neutral, add anhydrous Na 2 SO 4 dry. Remove the solvent with a rotary evaporator, pass through Al 2 o 3 column to obtain 1.23 g of a colorless oily product with a yield of 73%. The synthesis method of compound 2-19 is similar to that of compound 1. Compounds 1-19 1 H NMR, 13 C NMR...
Embodiment 2
[0032] Synthesis of compound 20:
[0033] In a 100mL three-neck flask equipped with a magnet, add 0.153g (22mmol) lithium, 1.680g (10mmol) dibenzofuran, 3.30mL (22mmol) tetramethylethylenediamine and 50mL dry diethyl ether, and heat to reflux for 24 hours . 1.402 g (8 mmol) of 1,3,3-trimethyl-2-indolinone was added at -78°C, the reaction was gradually raised to room temperature, and stirred overnight. Dry the solvent and tetramethylethylenediamine under reduced pressure, then add 50mL of diethyl ether, slowly add 3N dilute hydrochloric acid for hydrolysis, separate the layers, wash the organic phase with 3N dilute hydrochloric acid (20mL×3), combine the aqueous phase, and slowly add NaOH solution to neutral, a large amount of solids precipitated, filtered, and the obtained solids were washed with CH 2 Cl 2 Recrystallized with n-hexane to obtain 2.04 g of light pink crystals. Compound 20 1 H NMR, 13 See Tables 1-4 for CNMR, MS, EA, mp and yield.
Embodiment 3
[0035] Synthesis of compounds 21-32:
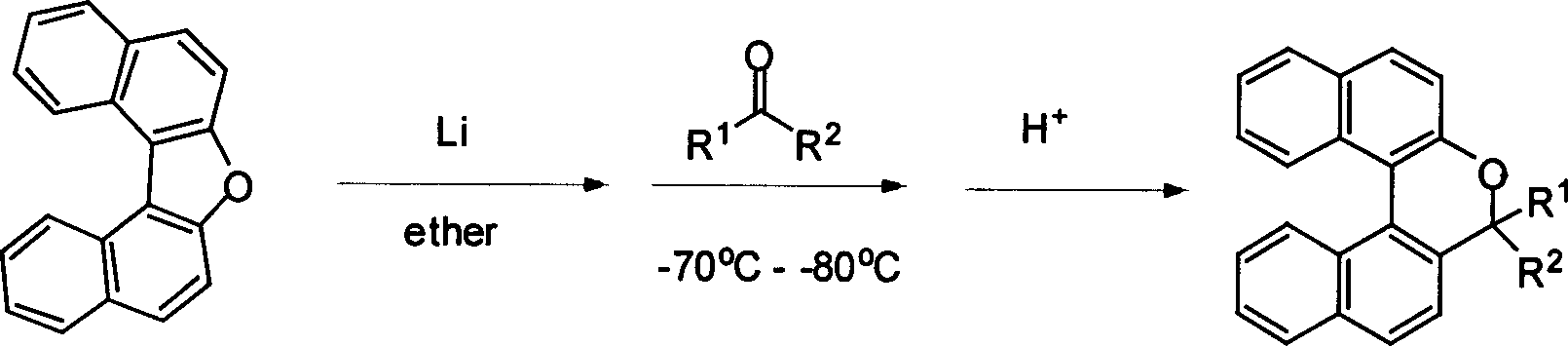
[0036] In a 100 mL three-neck flask equipped with a magnet, add 0.153 g (22 mmol) of lithium, 2.680 g (10 mmol) of dinaphthofuran and 50 mL of dry diethyl ether, and stir at room temperature for 24 hours. 0.59 mL (8 mmol) of acetone was added at -78°C, the reaction was gradually raised to room temperature, and stirred overnight. The next day, slowly add 3N dilute hydrochloric acid for hydrolysis, separate the liquid, add 20mL concentrated hydrochloric acid to the organic phase, stir at room temperature for 2 hours, separate the liquid, and wash the aqueous phase with CH 2 Cl 2 Extract (20mL×4), combine the organic phases, wash with water until neutral, add anhydrous Na 2 SO 4 dry. Then the solvent was removed by rotary evaporator, and the 2 o 3 column to obtain 1.79 g of a colorless solid with a yield of 72%. The synthesis method of compound 22-32 is similar to that of compound 21. Compounds 21-32 1 H NMR, 13 C NMR, MS, EA, mp a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com