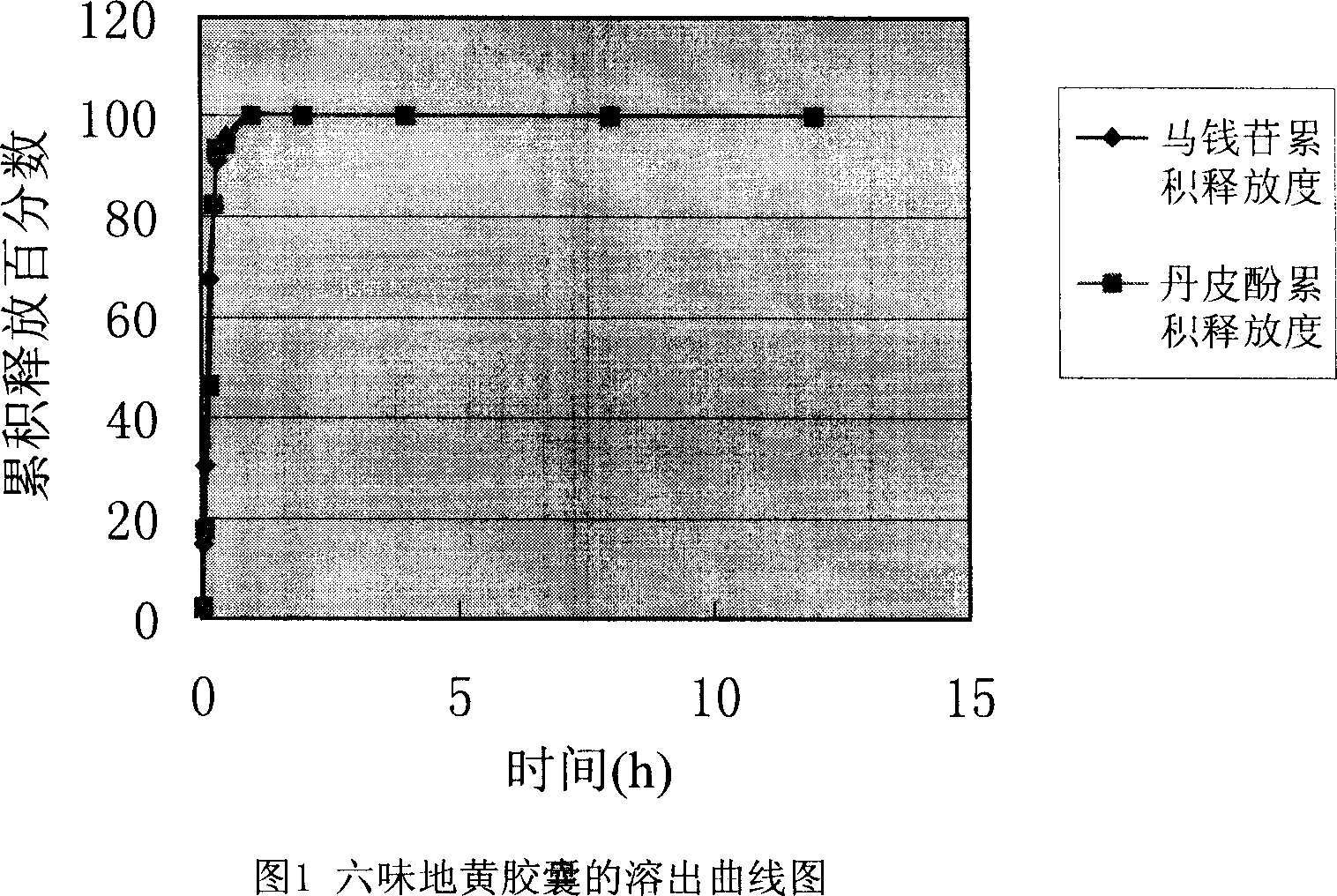
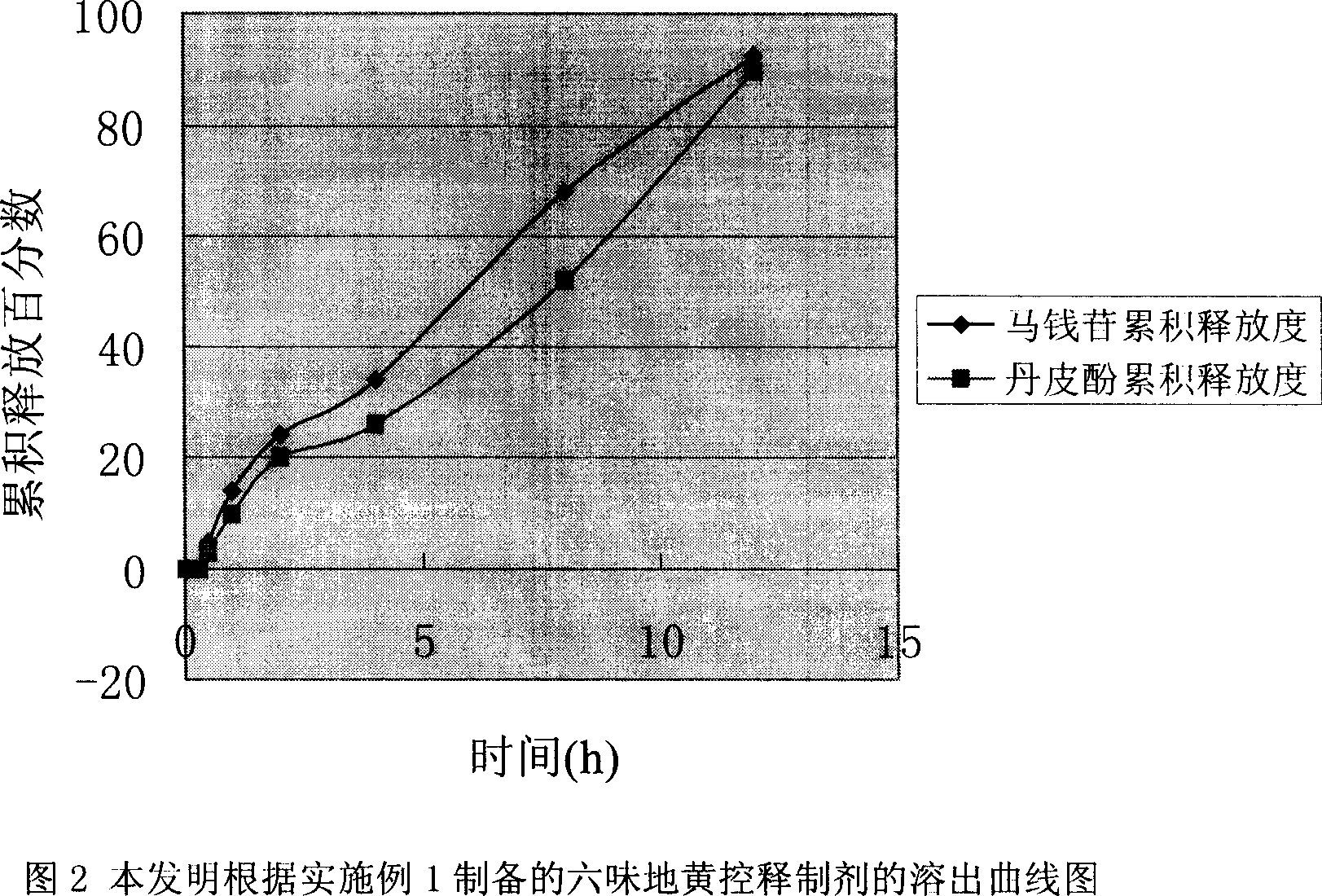
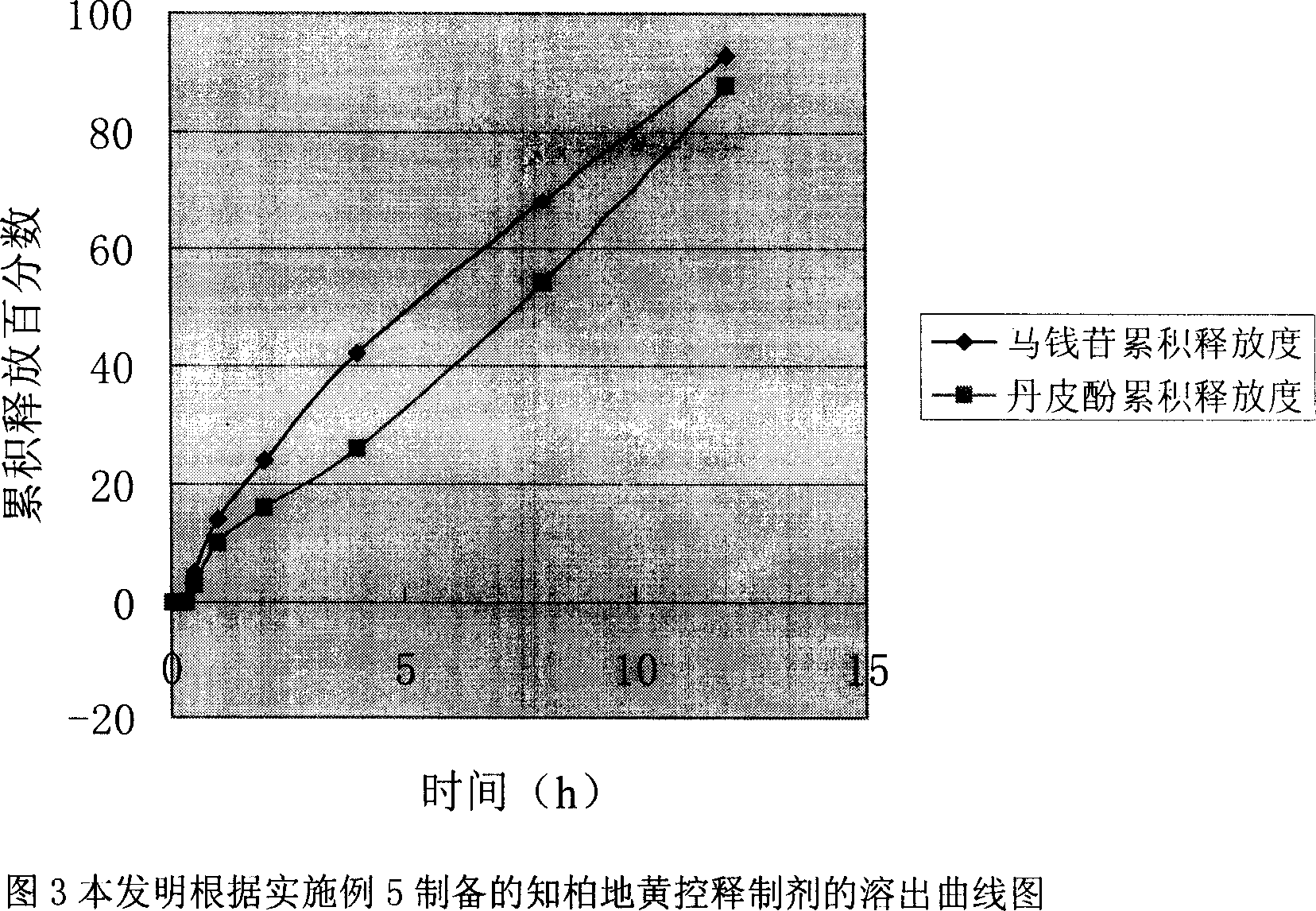
Osmotic pump type controlled release preparation of liuweidihuang or its adjusted formula extract and its preparing method
A technology of osmotic pump controlled release and Liuwei Dihuang, which is applied in the field of medicine, can solve the problems of few compound preparations of traditional Chinese medicine, unreported and complex ingredients, and achieve the effect of reducing the number of times of taking medicine, eliminating the phenomenon of peaks and valleys, and stable drug release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Chip
[0055] Drug-containing layer
[0056] Liuwei Dihuang Extract 300g
[0057] Polyoxyethylene N10 120g
[0058] Poloxamer F68 7.65g
[0060] 10% PVP absolute ethanol QS
[0061] booster layer
[0062] HPMC K4M 3g
[0063] Polyoxyethylene 303 70g
[0065] PVP K30 20g
[0066] Iron Oxide Red 1%
[0068] 10% PVP absolute ethanol QS
[0069] Coating solution prescription: cellulose acetate 15g
[0070] PE64000 0.53g
[0071] Acetone: water (97:3) 500ml
[0072] Preparation process: Mix the prescribed amount of medicine with polyoxyethylene N10, poloxamer F68, magnesium stearate, 10% PVP absolute ethanol is used as the binder to granulate, and dry to obtain granule A. HPMC K4M, polyoxyethylene Ethylene 303, sodium chloride, PVP K30, red iron oxide, mixed evenly, granulated with 10% PVP absolute ethanol as binder, dried to obtain granule B, added with lubricant, and pr...
Embodiment 2
[0085] Chip
[0086] Drug-containing layer
[0087] Liuwei Dihuang Extract 300g
[0088] Polyoxyethylene N10 120g
[0089] Poloxamer F68 15g
[0091] 10% PVP absolute ethanol QS
[0092] booster layer
[0093] HPMC K4M 3g
[0094] Polyoxyethylene 301 70g
[0096] PVPK30 20g
[0097] Iron Oxide Red 1%
[0098] Magnesium Stearate 1%
[0099] 10% PVP absolute ethanol QS
[0100] Coating solution prescription: cellulose acetate 15g
[0101] PEG40000.8g
[0102] Acetone: water (97:3) 500ml
[0103] Preparation process: same as before
Embodiment 3
[0105] Chip
[0106] Drug-containing layer
[0107] Liuwei Dihuang Extract 500g
[0108] Polyoxyethylene N10 120g
[0109] Poloxamer F68 20g
[0110] Magnesium Stearate 1%
[0111] 10% PVP absolute ethanol QS
[0112] booster layer
[0113] HPMC K4M 3g
[0114] Polyoxyethylene 303 50g
[0115] Sodium chloride 7g
[0116] PVPK30 20g
[0117] Iron Oxide Red 1%
[0118] Magnesium Stearate 1%
[0119] 10% PVP absolute ethanol QS
[0120] Coating solution prescription: cellulose acetate 15g
[0121] PEG40000.25g
[0122] Acetone: water (97:3) 500ml
[0123] Preparation process: same as before
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com