Fusion protein containing haman parathyroxin 1-34 and its expression vector
A parathyroid hormone and fusion protein technology, applied in the field of genetic engineering, can solve problems such as complex preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Design and artificial synthesis of DNA sequences expressing hPTH(1-34)
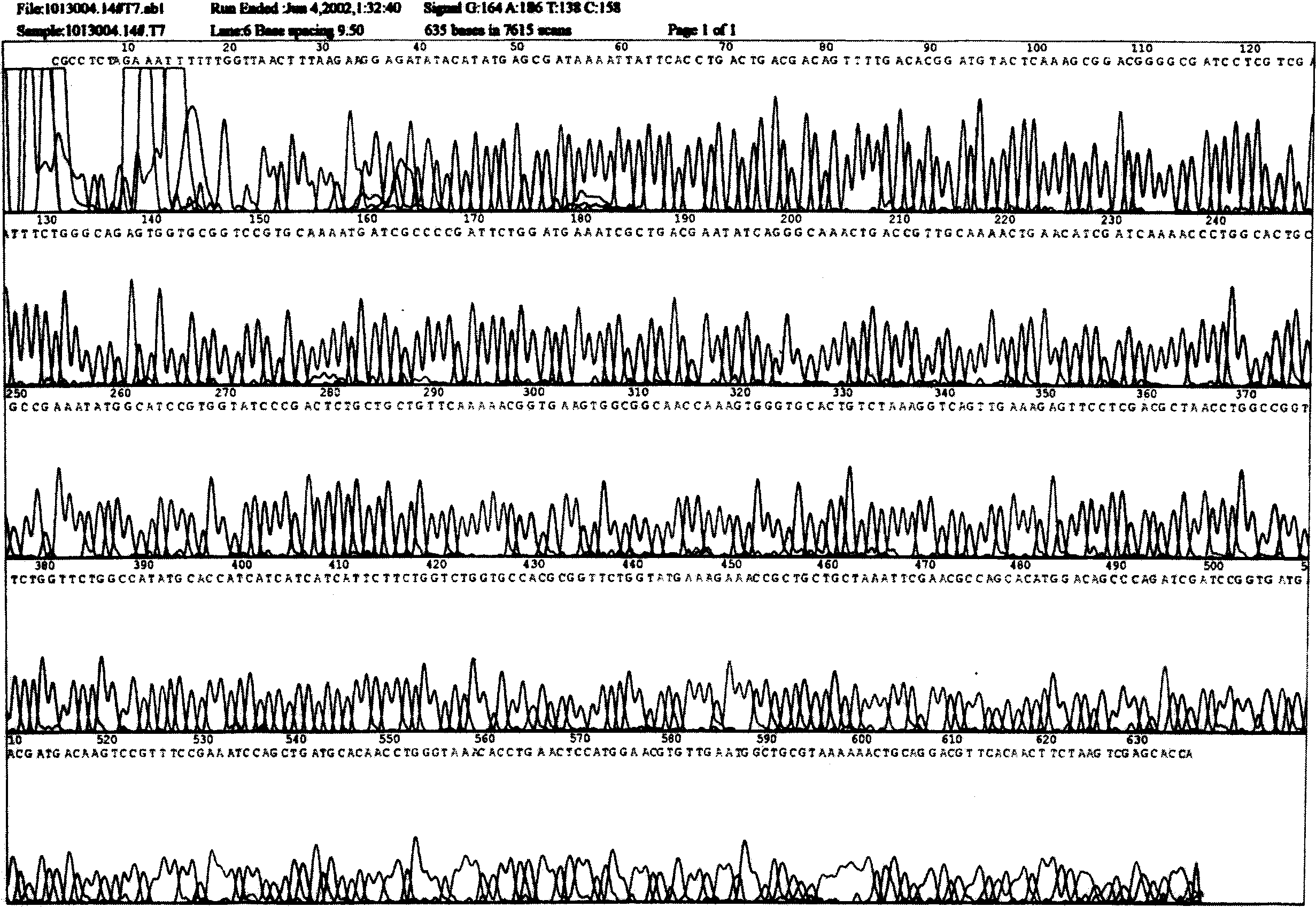
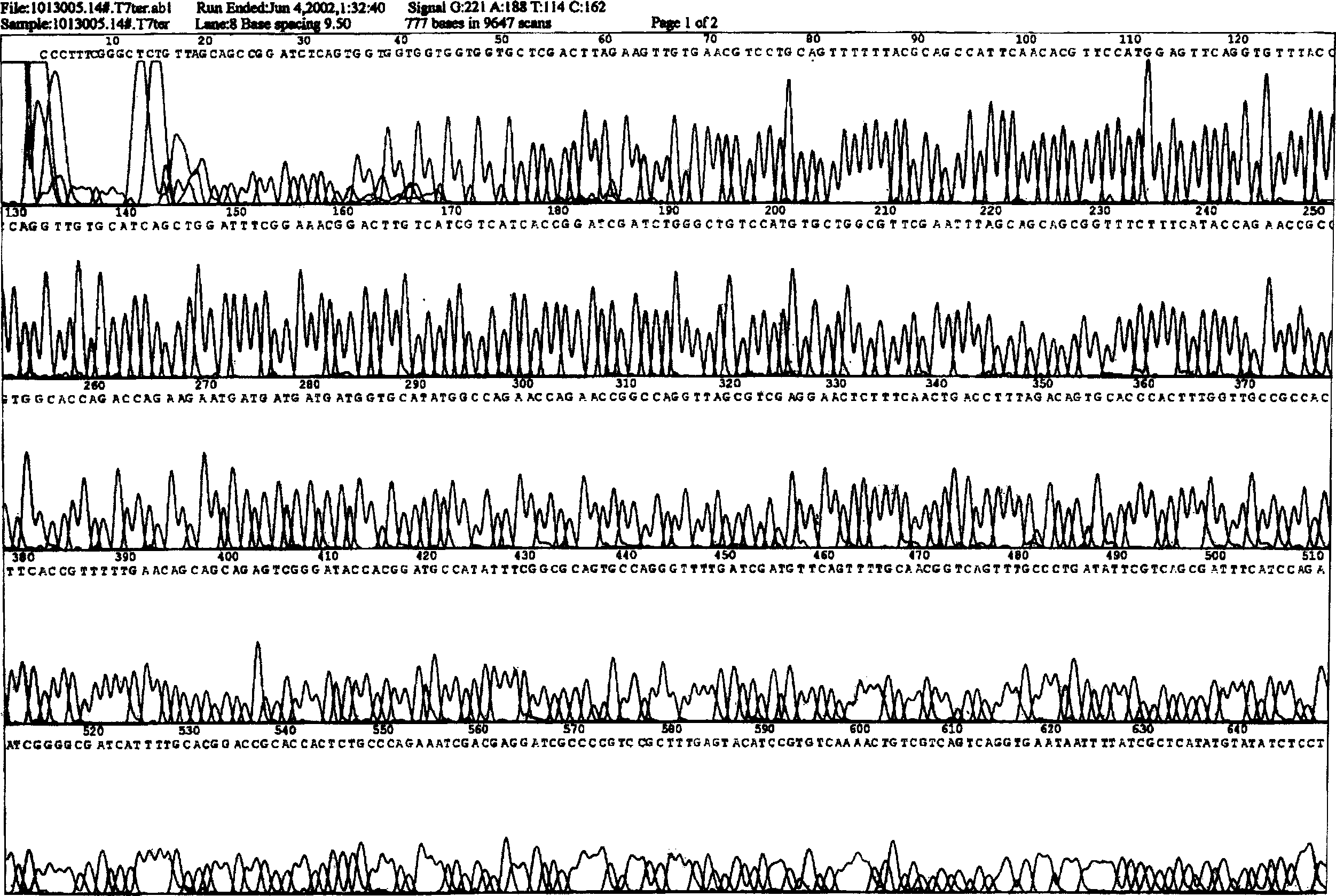
[0030] According to the amino acid sequence of hPTH (1-34) (see Table 1) and the codon preference of E. coli, an optimized DNA sequence suitable for expression in E. coli was synthesized artificially. When synthesizing hPTH(1-34) gene, KpnI restriction site GGTACC was introduced at the 5' end of the gene, stop codon TAA and SalI restriction site GTCGAC were introduced at the 3' end, and KpnI enzyme was introduced at the 5' end After the cleavage site, add the coding sequence GACGACGACGACAAG of the enterokinase cleavage recognition site to obtain sequence 1 (see Figure 10 ). To facilitate subsequent subcloning, the synthetic gene was cloned into pUC18 for preservation of the synthetic DNA sequence. Plasmid pUC18 contains the same KpnI and SalI restriction sites as plasmid pET32a(+).
[0031] serial number
[0032] Note: The amino acid sequence is marked from the N-terminus ...
Embodiment 2
[0033] Example 2 Construction process of recombinant plasmid
[0034] The following molecular cloning techniques, unless otherwise specified, refer to the literature: Molecular Cloning Experiment Guide (translated by Huang Peitang et al., [US] Sambrook et al., Science Press, 2002). The DNA extraction kit (UNIQ-10), DNA gel recovery kit (UNIQ-10) and ligation kit used in DNA manipulation were purchased from Shanghai Sangon Bioengineering Technology Service Co., Ltd. The cloning vector pUC18 and restriction enzymes were purchased from Fermentas LifeScience Company. Expression vector pET32a(+), E. coli TOP10 and BL21(DE3) were purchased from Novagen Company. The E. coli host for cloning is TOP10, and the E. coli host for expression is BL21 (DE3). The BL21(DE3) genotype was: hsdS gal (λcIts857ind1 Sam7 nin5 lacUV5-T7 geneI). BL21(DE3) carries the T7RNA polymerase gene. Under the induction of IPTG, a large amount of T7RNA polymerase is produced, thus enabling the expression of e...
Embodiment 3
[0057] Example 3 Induced expression of engineering bacteria
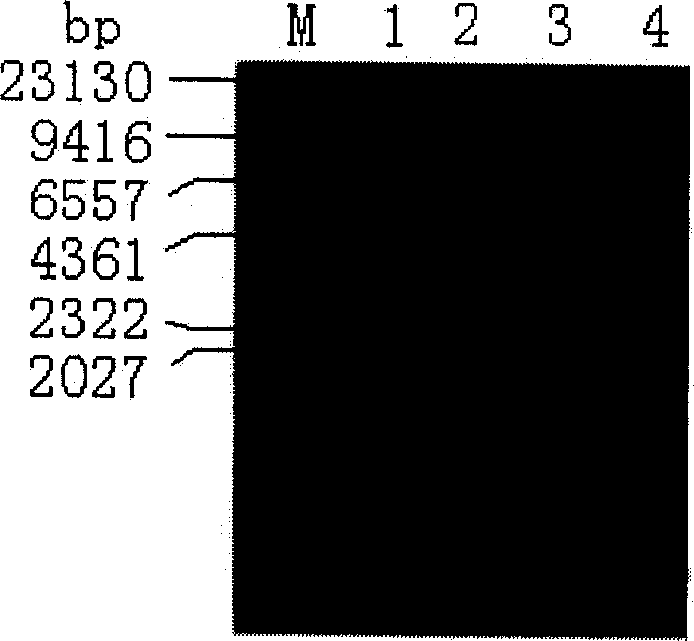
[0058] Escherichia coli BL21(DE3) is transformed with the recombinant plasmid pET-PTH(1-34) DNA, and the obtained is the genetically engineered bacteria for expressing the rhPTH(1-34) fusion protein. Pick 8 single colonies, culture overnight at 37°C in 5ml LB medium (1% peptone, 0.5% yeast extract, 1% NaCl) containing 100μg / ml Amp, transfer to 50ml containing 100μg / ml Amp cultured in LB medium at 37°C, and the remaining bacterial solution was subpackaged with 15% glycerol and frozen. OD 600 When it reached 0.5, IPTG was added to a final concentration of 0.5mM to induce expression, and samples were taken for SDS-PAGE electrophoresis after 4 hours. Compared with the uninduced control, the induced recombinants all had an expression band with an expected molecular weight of about 20 kDa, and the expression amount was more than 25% of the total bacterial protein. Bacteria No. 6 with the highest yield was taken as the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com