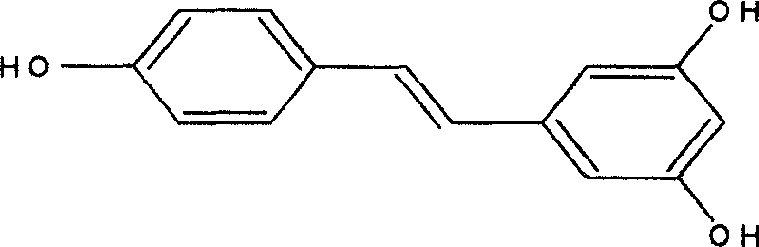
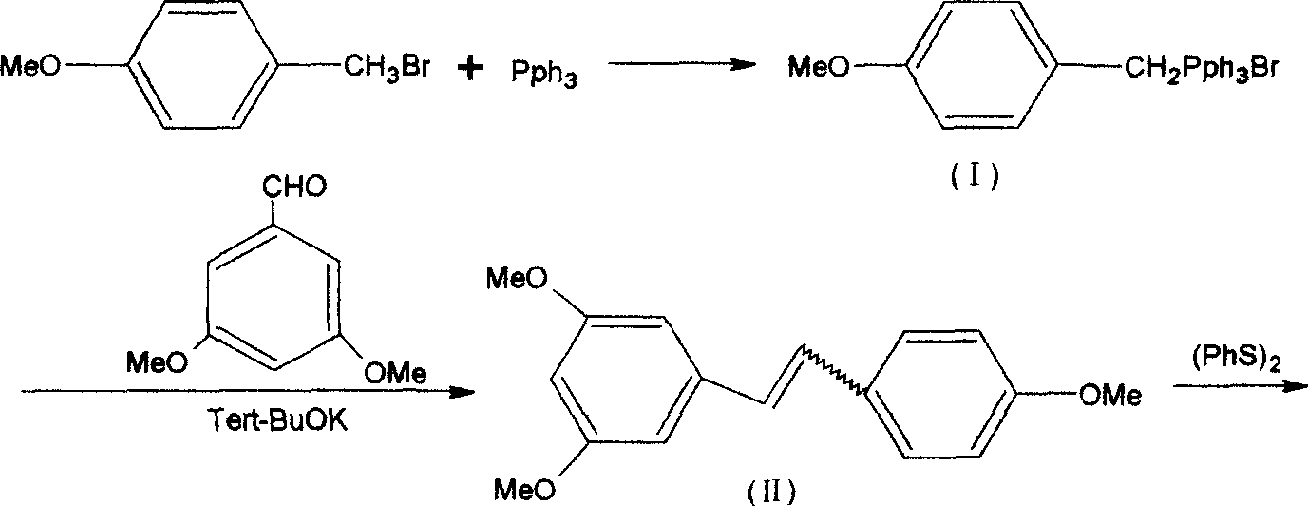
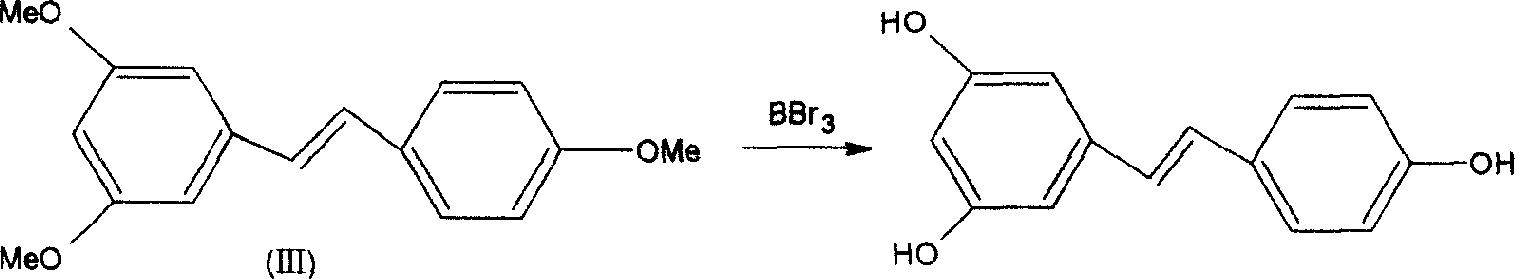
Method for synthesizing veratric alcohol
A synthesis method and technology of resveratrol are applied in chemical instruments and methods, preparation of organic compounds, organic chemistry and other directions, can solve the problems of difficult conversion, harsh reaction conditions, waste of solvents and products, etc. The effect of mild reaction conditions and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Step A: Take 20g of p-methoxybenzyl alcohol and 100ml of benzene in a three-necked round bottom flask equipped with a condenser, stir, water bath, and evenly and quickly introduce HBr gas, and the solution will change from turbidity to transparent, and then heat to 58℃ , React for about 0.5 hours, wash 300ml×4 with water, use anhydrous MgSO for organic phase 4 After drying and rotary evaporation to remove benzene, 20.81 g of p-methoxybenzyl bromide was obtained as a yellow oil (yield 71.4%), which was directly used in the next reaction.
[0024] Step B: 19.75g of p-methoxybenzyl bromide (unpurified) is reacted with 56ml of triethyl phosphite at 90-100°C for 3 hours, and the triethyl phosphite is removed under reduced pressure by rotating to obtain p-methoxybenzyl 36.98g of diethyl phosphate was directly used in the next reaction.
[0025] Step C: 36.98g p-methoxybenzyl diethyl phosphate (unpurified) was placed in 250ml dry DMF, cooled to below 0°C in an ice bath, stirred qu...
Embodiment 2
[0028] Step A: Take 20g of p-methoxybenzyl alcohol and 100ml of benzene in a three-necked round bottom flask equipped with a condenser, stir, water bath, and evenly and quickly introduce HBr gas, the solution can be changed from turbid to transparent, and then heated to 50°C , React for about 1 hour, wash 300ml×4 with water, use anhydrous MgSO for organic phase 4 After drying, the benzene was removed by rotary evaporation to obtain p-methoxybenzyl bromide as a yellow oil, which was directly used in the next reaction.
[0029] Step B: 19.75g of p-methoxybenzyl bromide (unpurified) is reacted with 56ml of triethyl phosphite at 90-100°C for 2h, and the triethyl phosphite is removed under reduced pressure by rotating to obtain p-methoxybenzyl 35.08g of diethyl phosphate was used directly in the next reaction.
[0030] Step C: 36.98g p-methoxybenzyl diethyl phosphate (unpurified) was placed in 250ml dry DMF, cooled to below 0°C in an ice bath, stirred quickly, and added 24.55g NaOCH 3 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com