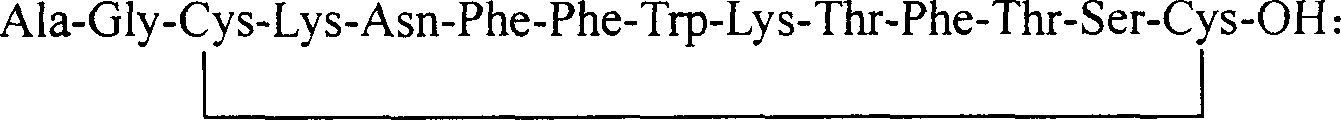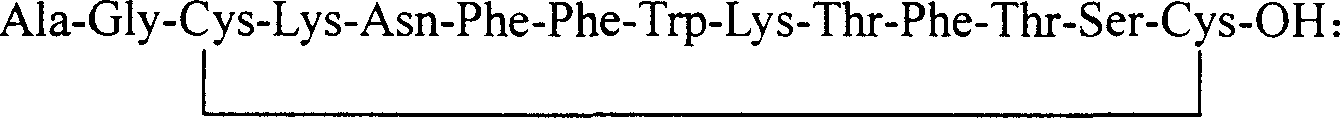Preparation method of synthesizing growth chalone from solid phase polypeptide
A solid-phase peptide synthesis, somatostatin technology, applied in the fields of peptide preparation, somatostatin, chemical instruments and methods, etc., can solve the problems of low column efficiency, environmental pollution, cost increase and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] In the example:
[0118] Said peptide-connecting reagent is: NMM:DMF=1:10, volume ratio;
[0119] Said decapping reagent is: PIP: DMF=1: 3.5, volume ratio;
[0120] synthetic peptide chain
[0121] (1) Preparation of Fmoc-Cys(Trt)-resin:
[0122] Weigh 50g of trityl resin (100-400 mesh, 1.0mmol / g), soak in 500ml of DMF for 30 minutes to fully swell the resin, add 44ml of DIPEA, 87.9g of Fmoc-Cys(Trt)-OH, and react at 25°C 1 hour. Add 20 ml of methanol and react at 25°C for 1 hour. Blow dry with nitrogen, wash the resin three times with DMF, and blow dry with nitrogen.
[0123] (2) Preparation of Fmoc-Ser(tBu)-Cys(Trt)-resin:
[0124] In the Fmoc-Cys(Trt)-resin of step (1), add 500ml of decapping reagent, react for 30 minutes at 25°C, blow dry, wash with DMF, blow dry, add Fmoc-Ser(tBu )-OH 57.5g, TBTU 48.1g and HOBT 20.2g mixture, reacted at 25°C for 2 hours, dried, washed with DMF and ethanol respectively, and dried to obtain Fmoc-Ser(tBu)-Cys(Trt)-resin;...
Embodiment 2
[0162] Adopt the same method and processing condition as embodiment 1, wherein:
[0163] Use 4-methoxytrityl resin as the starting material, connect Fmoc-Cys(Trt)-OH with the same method as before, then add decapping reagent, react at 25°C for 0.5 hours, add Fmoc dissolved in peptide reagent -The mixture of amino acid, TBTU / HBTU and HOBT was reacted at 25°C for 2 hours, and then subjected to reactions such as peptide cutting and oxidation to obtain 14.8g of white loose block finished product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com