Novel use of 2(2-hydroxypropyl) phenol
A technology of hydroxypropyl and phenol, which is applied in the new application field of 2(2-hydroxypropyl)phenol, can solve the problems of short drug duration and other problems, and achieve the effect of simple preparation method, broad application prospect and stable chemical structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] The preparation of embodiment 1,2 (2-hydroxypropyl) phenol
[0011] 2(2-hydroxypropyl)phenol can be synthesized from allylphenol, and the reaction formula is as follows:
[0012]
[0013] A 250mL three-necked flask was equipped with a thermometer, a reflux condenser and a constant pressure dropping funnel, and 13.4g (0.1mol) of 96% o-allylphenol and 20mL of ethyl acetate were added to the three-necked flask, and a magnetic stir bar was added. Add 50mL of 50% sulfuric acid into the separatory funnel, start the electromagnetic stirrer, add sulfuric acid dropwise to the reaction bottle under cooling in an ice bath, remove from the ice bath after the dropwise addition, and stir at room temperature for 1 h.
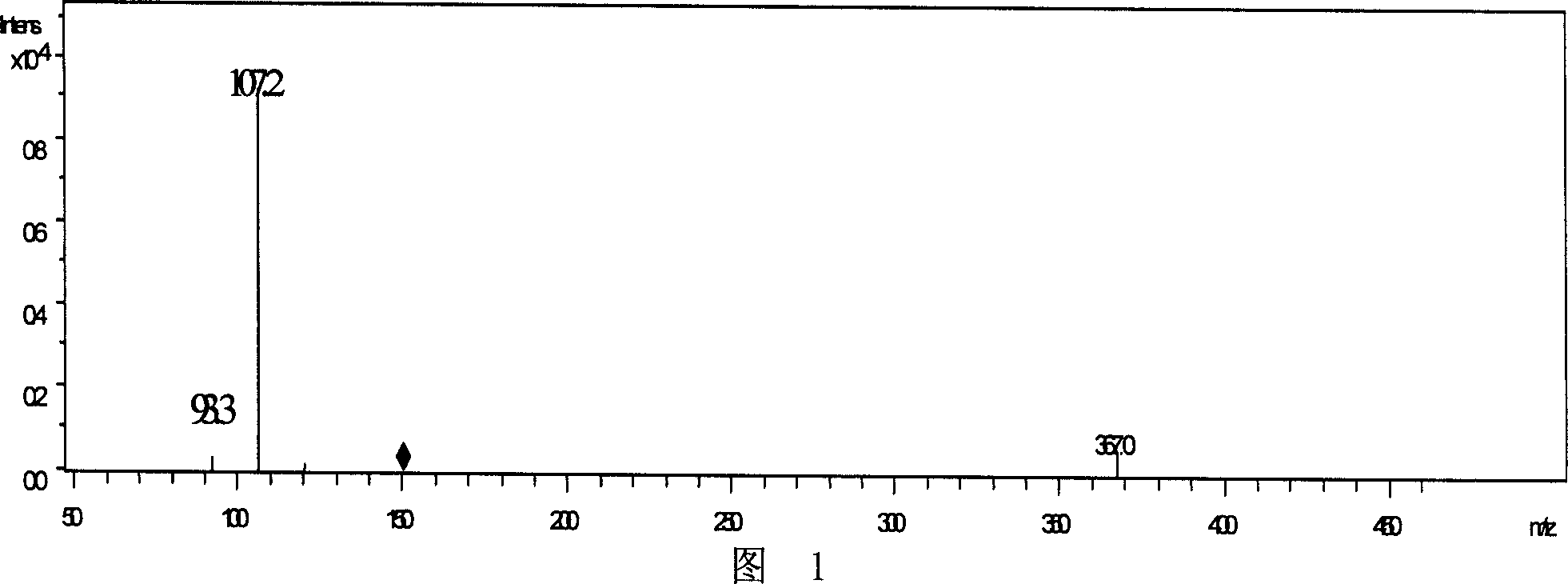
[0014] The synthesized product was separated and purified by column chromatography (filler was silica gel), and detected by mass spectrometry, the molecular ion peak was 150.9, and the ion fragment peaks were 107.1 and 93.3, respectively. The mass spectrum is shown i...
Embodiment 2
[0017] The biological activity of embodiment 2,2 (2-hydroxypropyl) phenol
[0018] Effect of 2(2-hydroxypropyl)phenol on wheat sheath blight (Rhizoctonia cerealis), melon and fruit rot fungus (Rhizoctonia cerealis), tomato gray mold (Botrytis cinerea) and apple rot fungus (Valsa mali) by growth rate method The above strains were all selected from commonly used commercial strains.
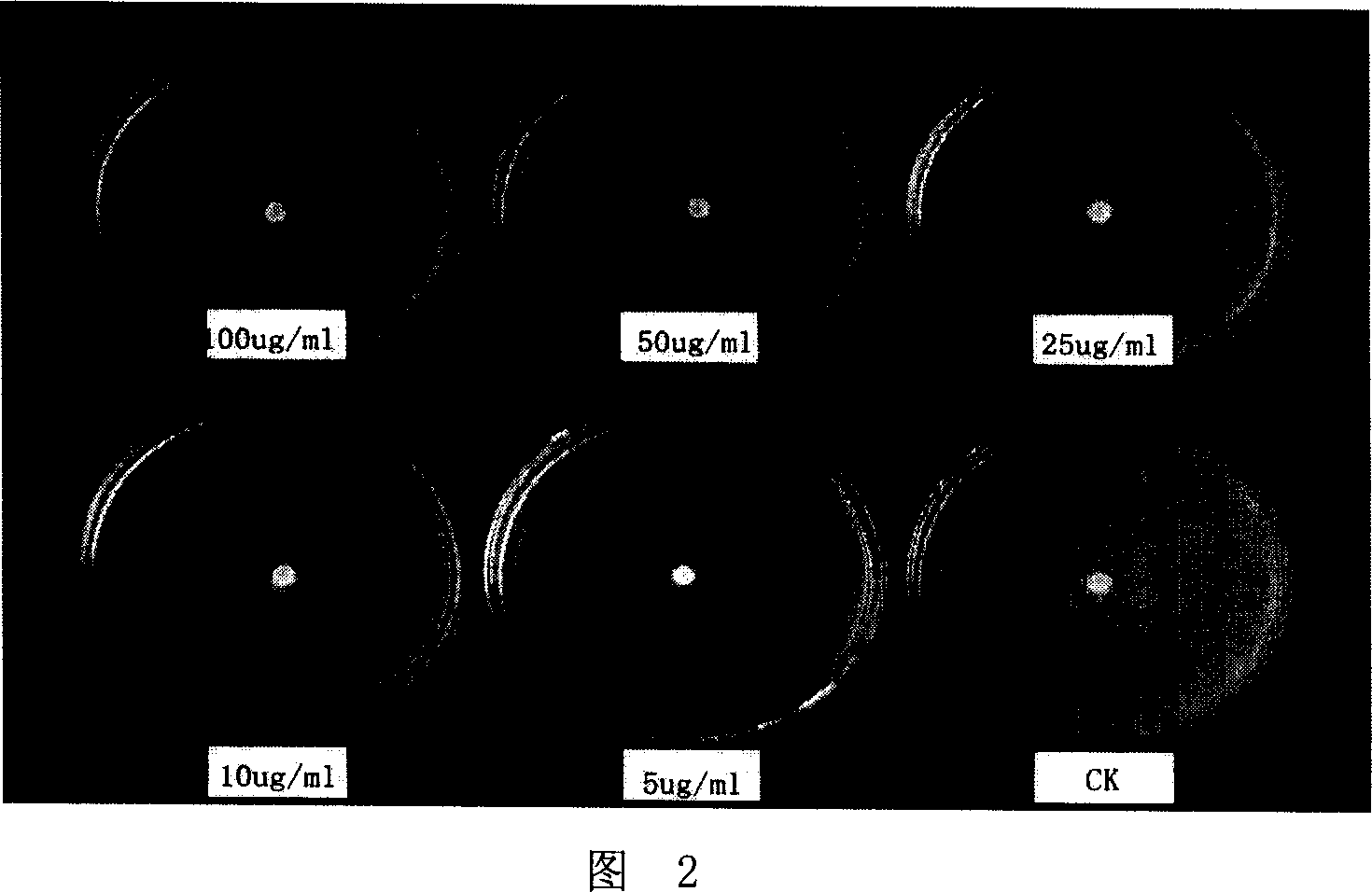
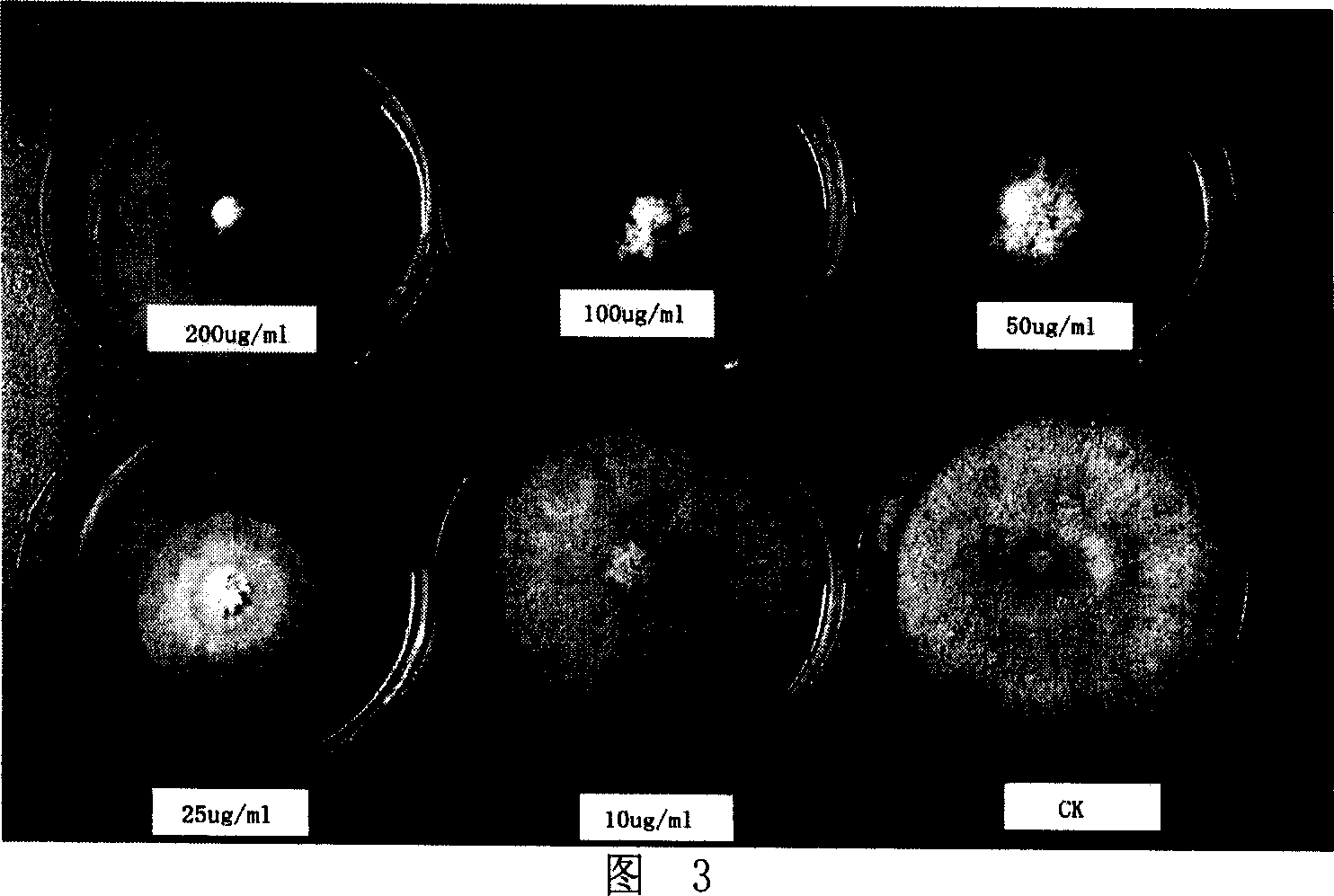
[0019] Figure 2-Figure 5 are photos of the inhibitory effect of different concentrations of 2 (2-hydroxypropyl) phenol on Rhizoctonia acerealis (concentrations are respectively 0, 5, 10, 25, 50, 100 μg·mL -1 ); apple rot pathogen (Valsa mali) (concentrations were 0, 10, 25, 50, 100, 200 μg·mL -1 ); tomato gray mold (Botrytiscinerea) (concentrations were 0, 5, 10, 25, 50, 75 μg·mL -1 ); wheat sheath blight (Rhizoctonia cerealis) (concentrations were 0, 1, 5, 10, 25, 50 μg·mL -1 ).
[0020] The antibacterial rate was calculated according to the following formula:
[0021]
[0022] Convert the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com