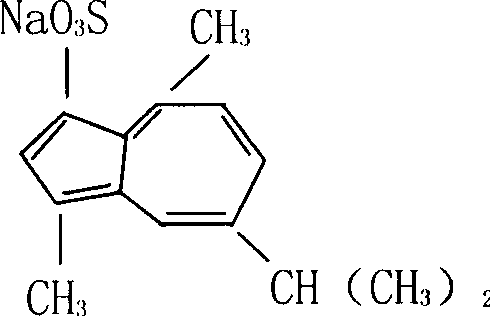
Film agent comprising sodium gualenate using for eyes
An azulene sulfonate sodium, ophthalmic technology, applied in the field of ophthalmic films, can solve the problems of use limitation, high viscosity, blocking sight, etc., and achieve the effects of stable properties, long effective concentration and convenient use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] The preparation method of the ophthalmic film containing sodium azulene sulfonate of the present invention is: take the film forming agent and glycerin according to the prescription, add an appropriate amount of water for injection and soak for 3 to 24 hours, after the film forming agent is completely soaked and expanded, heat it on a water bath Dissolve at 80°C to make a film-forming material slurry for use. In addition, weigh sodium azulene sulfonate according to the prescription amount and add sterilized water for injection and heat it in a water bath to dissolve it, add it into the slurry of the dissolved film-forming material and stir evenly, put it in a water bath at 80°C±5 for 30 minutes, and then pour it into the pre- Hand-made film 2000cm on a glass plate coated with a small amount of liquid paraffin 2 , after 1 hour blast drying at 70-80°C, remove the film immediately, take it out and sterilize it under ultraviolet light for 30 minutes, cut into 0.5×1cm 2 Sma...
Embodiment 1
[0023] Prescription: sodium azulene sulfonate 0.1g; polyvinyl alcohol 30g; glycerin 5ml; sterilized water 100ml; 2 , cut into 0.5×1cm 2 Small blue pieces, aseptically packaged.
[0024] Operation method: Weigh 30g of polyvinyl alcohol and 5ml of glycerin, add 50ml of sterilized water and soak for 24 hours, make the polyvinyl alcohol completely infiltrate and swell, then heat to 80°C on a water bath to dissolve, and make a film-forming material slurry. Take another 0.1 g of sodium azulene sulfonate, add 50 ml of sterilized water, heat it on a water bath to dissolve it, add it into the slurry of the dissolved film-forming material, stir well, put it in a water bath at 80 ° C ± 5 for 30 minutes, and then pour it into A glass plate pre-coated with 2g of liquid paraffin, hand-made film 2000cm 2 , after 1 hour blast drying at 70-80°C, remove the film immediately, take it out and sterilize it under ultraviolet light for 30 minutes, cut into 0.5×1cm 2 Small blue pieces, aseptically...
Embodiment 2
[0026] Prescription: sodium azulene sulfonate 0.4g; hydroxypropyl cellulose 35g; glycerin 6ml; sterilized water 100ml; 2 , cut into 0.5×1cm 2 Small pieces, sterile packaged.
[0027] Operation method: Weigh 35g of hydroxypropyl cellulose, 6ml of glycerin, add 50ml of sterilized water and soak for 4 hours, make the polyvinyl alcohol completely infiltrate and swell, then heat to 80°C on a water bath to dissolve, and make a film-forming material slurry. Take another 0.4 g of sodium azulene sulfonate, add 50 ml of sterilized water, heat it on a water bath to dissolve it, add it into the slurry of the dissolved film-forming material, stir well, put it in a water bath at 80 ° C ± 5 for 30 minutes, and then pour it into Hand-made film 2000cm on a glass plate pre-coated with 2g glycerin 2 , after 1 hour blast drying at 70-80°C, remove the film immediately, take it out and sterilize it under ultraviolet light for 30 minutes, cut into 0.5×1cm 2 Small tablets, sterile packaged.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com