Process for biochemical production of glyoxylic acid
A technology of glyoxylic acid and microorganisms, applied in the field of glyoxylic acid preparation, can solve the problems of unreported oxidation of glyoxal oxidase, difficulty in transformation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Liquid culture medium (EG-NB medium ( pH 7)) 5ml was poured into a large test tube and autoclaved at 121°C for 20 minutes. Under sterile conditions, the culture medium was inoculated with the microorganisms shown in Table 3 using an inoculation loop, and cultured at 28° C. for 2 days to obtain a pre-culture solution. Next, 1 ml of the obtained preculture solution was inoculated into 100 ml of sterilized EG-NB medium in a 500 ml capacity Sakaguchi flask, and cultured at 28° C. for 3 days. 100 ml of the obtained culture solution was centrifuged to collect cells, washed with 100 mM Tris-HCl buffer (pH 8.0), and suspended in 5 ml of the same buffer (pH 8.0). The cell suspension was disrupted with a micro-oscillating impactor (manufactured by BIOSPEC), and then centrifuged to obtain a supernatant (cell-free extract). Add 0.1 ml of 500 mM glyoxal aqueous solution and 0.1 ml of 50,000 U / ml catalase solution to 0.8 ml of the obtained cell-free extract, and shake the reaction ...
Embodiment 2
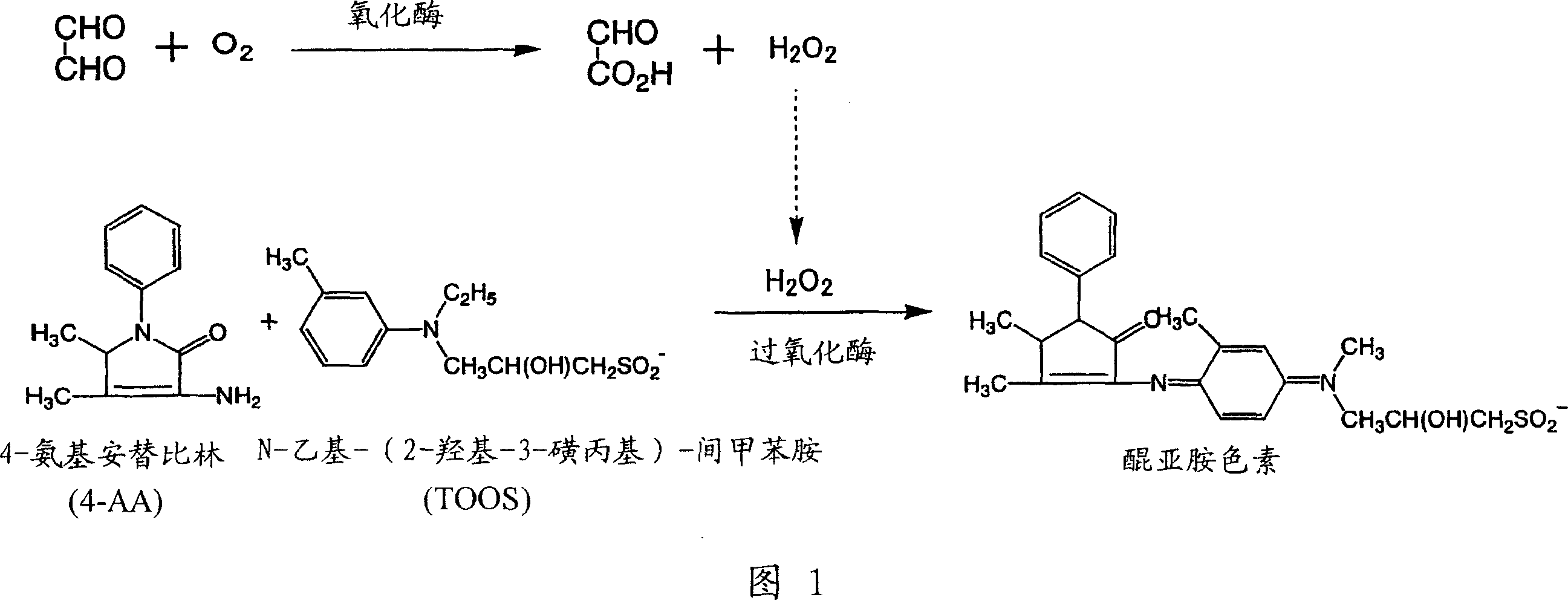
[0112] In the microbial cell-free extract solution described in Table 3 prepared in 0.1ml of Example 1, add 0.1M phosphate buffer (pH7) 0.05ml containing 4-AA 1.34mM, TOOS 2.19mM, POD6U / ml in a test tube . Then add 100 mM glyoxal aqueous solution or 0.05 ml of water, shake at 28° C. for 2 minutes, and observe the color change of the reaction solution. The results are shown in Table 4. In all the reactions using any of the above-mentioned microorganisms, the color of the reaction solution in the test in which the glyoxal aqueous solution was added was deep purple, but when water was added instead of the glyoxal aqueous solution, the reaction solution did not change color. It was found that hydrogen peroxide was generated during the oxidation reaction of glyoxal, and thus it was found that the enzyme catalyzing the oxidation reaction of glyoxal was an oxidase.
[0113]
Embodiment 3
[0115]100ml of the culture solution of Pseudomonas KNK254, Microbacterium KNK011, Cellulomonas turbidity IFO15015 and Cellulomonas JCM2471 prepared in the same way as in Example 1 were collected by centrifugation and the cells were collected with 0.1mM phosphoric acid After washing with a buffer (pH 7), the suspension was suspended in 5 ml of the same buffer. In a test tube, 0.05 ml of a 500 mM glyoxal aqueous solution was added to 0.45 ml of the cell suspension, and the reaction was performed by shaking for 4 hours. The supernatant after the reaction was analyzed by HPLC to calculate the glyoxylic acid produced. As a result, 20 mM glyoxylic acid was produced in Pseudomonas sp. KNK254, 14 mM glyoxylic acid was produced in Microbacterium sp. 33 mM glyoxylate was produced in the sp. JCM2471.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com