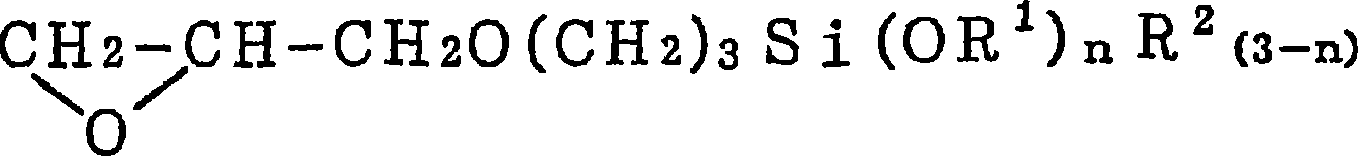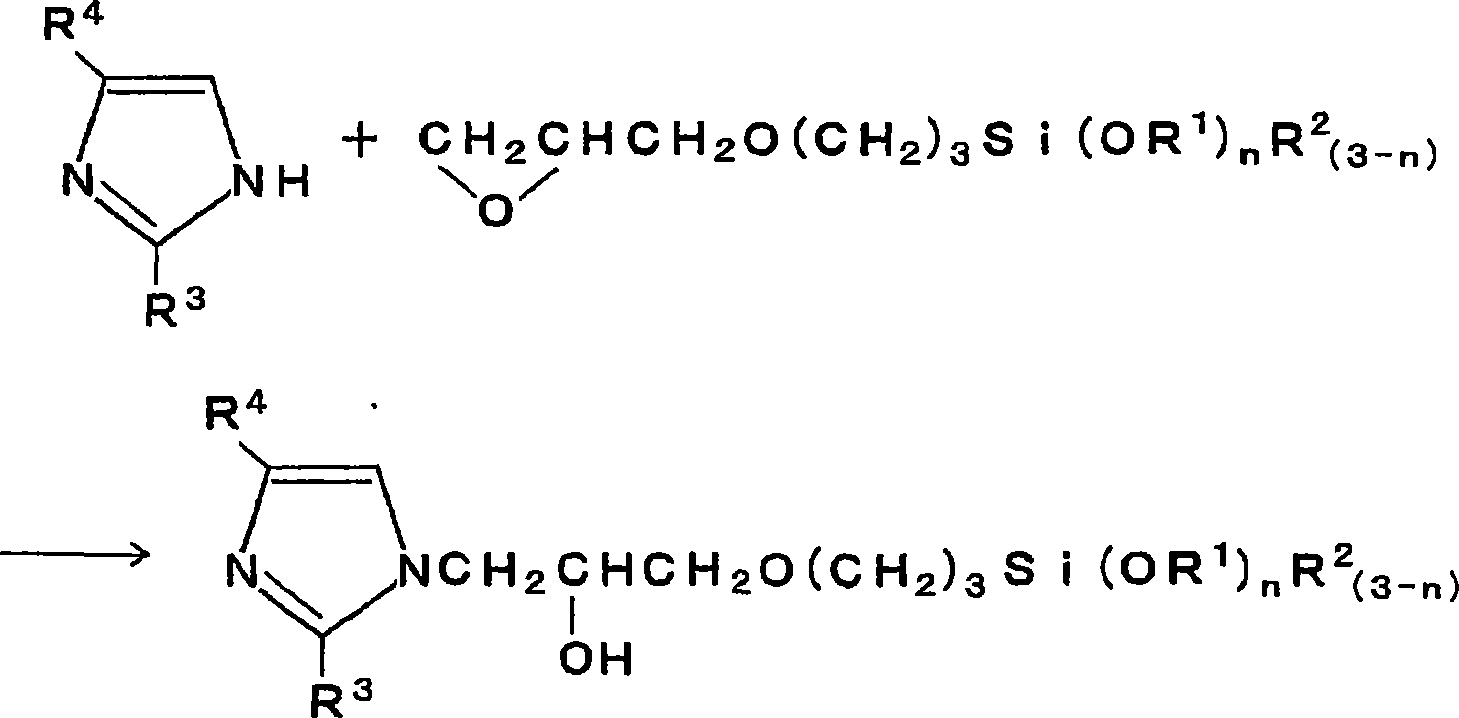Electroless copper plating solution and method for electroless copper plating
An electroless copper plating, solution technology, applied in circuits, electrical components, liquid chemical plating, etc., can solve problems such as difficult uniform plating
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] In a 0.16% by weight solution of a silane coupling agent as an equimolar reaction product of imidazole and γ-glycidoxypropyltrimethoxysilane, an aqueous solution of palladium chloride was added to reach 50 mg / liter, thereby preparing For the pretreatment agent for plating, the above-mentioned silicon wafer with the tantalum nitride film was immersed in the pretreatment agent at 50° C. for 5 minutes. Thereafter, the silicon wafer was heat-treated at 200°C for 15 minutes, followed by electroless copper plating at 60°C for 5 minutes. The composition of the plating solution is copper sulfate 0.04 mol / liter, ethylenediamine tetraacetate 0.4 mol / liter, formalin 0.1 mol / liter, sodium hypophosphite 0.1 mol / liter and 2,2'-bipyridine 10 mg / L, and the pH value is 12.5 (pH regulator: sodium hydroxide). A plated film was formed uniformly and without unevenness on the entire surface with a film thickness of 50 nm.
Embodiment 2
[0048] The silicon wafer with the tantalum nitride film was pretreated in the same manner as in Example 1, and then the silicon wafer was electrolessly copper-plated at 60° C. for 5 minutes. The composition of the plating solution is 0.04 mol / liter of copper sulfate, 0.4 mol / liter of ethylenediamine tetraacetate, 0.1 mol / liter of glyoxylic acid, 0.1 mol / liter of hypophosphorous acid and 10 mg / liter of 2,2'-dipyridyl rise, and the pH value is 12.5 (pH regulator: potassium hydroxide). A plated film was formed uniformly and without unevenness on the entire surface with a film thickness of 50 nm.
Embodiment 3
[0050] The silicon wafer with the tantalum nitride film was pretreated in the same manner as in Example 1, and then the silicon wafer was electrolessly copper-plated at 60° C. for 5 minutes. The composition of the plating solution is copper sulfate 0.04 mol / liter, ethylenediamine tetraacetate 0.4 mol / liter, formalin 0.1 mol / liter, ammonium hypophosphite 0.1 mol / liter and 2,2'-bipyridine 10 mg / L, and the pH value is 12.5 (pH regulator: sodium hydroxide). A plated film was formed uniformly and without unevenness on the entire surface with a film thickness of 50 nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com