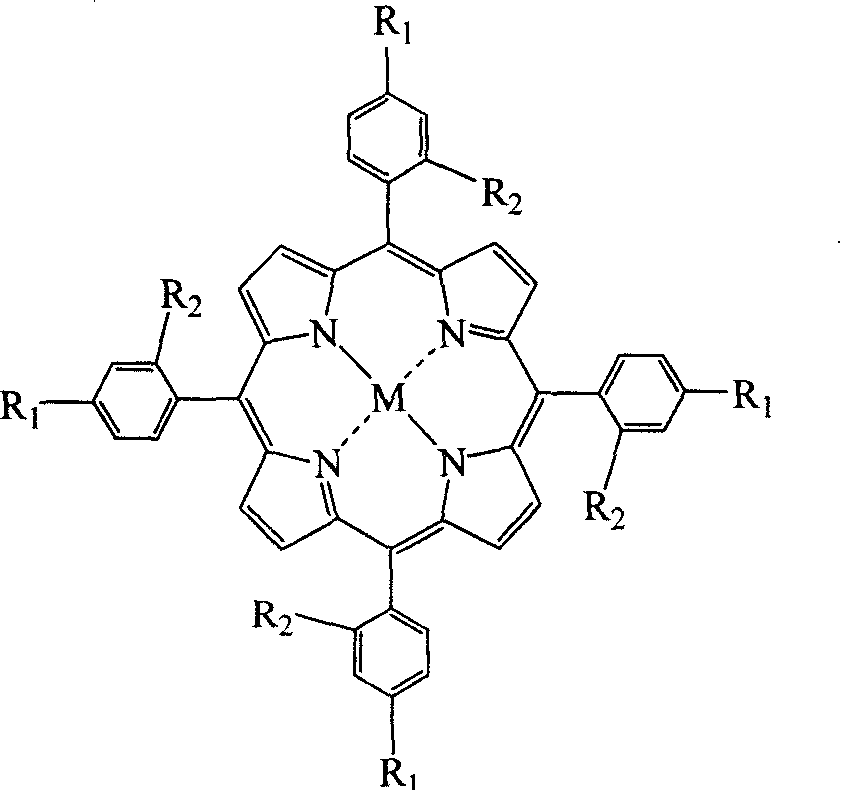
Synthetic method for metal porphyrin
A synthesis method and metalloporphyrin technology are applied in chemical instruments and methods, compounds of Group 7/17 elements of the periodic table, compounds containing elements of Group 8/9/10/18 of the periodic table, etc., and the steps are simple. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] In a 250mL three-necked flask, add 60mL propionic acid, 20mL glacial acetic acid and 20mL nitrobenzene (the weight percentage of the three is respectively 57%, 20%, 23%) mixed solvent, then add 10mmol benzaldehyde and 10mmol pyrrole, after , add 0.7g manganese acetate, heat to reflux for 1h, cool, add 30mL of methanol, stir, let stand overnight, and suction filter to get tetraphenylmanganese porphyrin (i.e. R in general formula (I) 1 = H, R 2 =H, M=Mn), weighed after drying, the yield was 57.6%, and its purity was 99.7% as detected by liquid chromatography.
Embodiment 2
[0018] In a 250mL three-necked flask, add 60mL propionic acid, 20mL glacial acetic acid and 20mL m-nitrotoluene (the weight percentage of the three is respectively 58%, 20%, 22%) mixed solvent, then add 10mmol p-nitrobenzaldehyde and 10mmol pyrrole, after that, add 0.7g ferrous acetate, heat and reflux 1h, cool, add 30mL methanol, stir, stand overnight, suction filtration, get tetra-(p-nitrophenyl)iron porphyrin (being general formula ( I) Medium R 1 = NO 2 , R 2 =H, M=Fe), weighed dry, its yield was 41.5%, and its purity was 99.8% as detected by liquid chromatography.
Embodiment 3
[0020] In the 250mL three-necked flask, add 70mL n-hexanoic acid and 30mL formic acid (the percentage by weight of the two is respectively 65%, 35%) mixed solvent, then add 10mmol p-chlorobenzaldehyde and 10mmol pyrrole, after that, add 0.7g copper acetate, Heated to reflux for 1h, cooled, added 30mL of methanol, stirred, left to stand overnight, and suction filtered to obtain tetra-(p-chlorophenyl)copper porphyrin (i.e. R in general formula (I) 1 = Cl, R 2 =H, M=Cu), weighed dry, its yield was 53.4%, and its purity was 99.9% as detected by liquid chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com