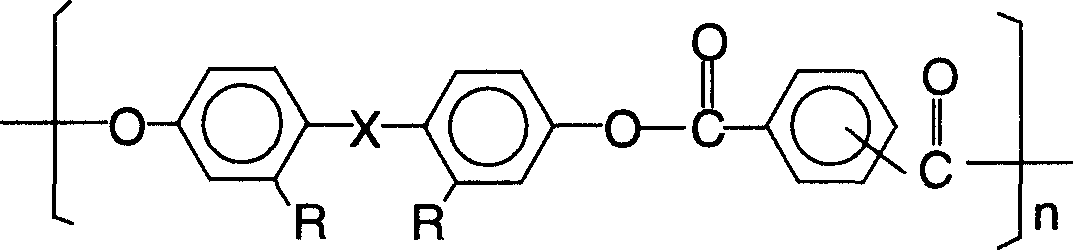
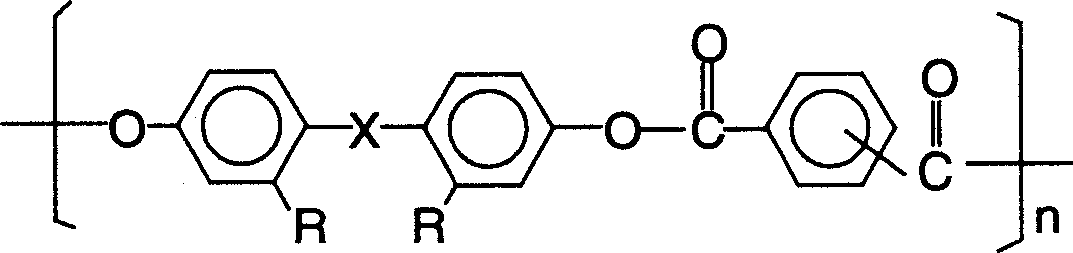
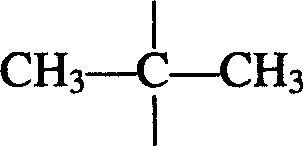
Bisphenol type poly arylate and its preparing method and use
A polyarylate and bisphenol-type technology, which is applied in the field of bisphenol-type polyarylate and its preparation, can solve the problems of increasing surface contact resistance, reducing the protective film of silver-plated electrical components, reducing solderability, etc., and achieving saving Energy, small impact, good mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: 946 parts of distilled water, 20 parts of sodium hydroxide, 45.8 parts of 2.2-bis(4-hydroxyphenyl)propane (bisphenol A), and 0.32 parts of triethylbenzyl ammonium chloride were added to a stirring In a three-necked flask with a thermometer and a thermometer, the water phase is formed after stirring and dissolving; the oil phase composed of 315 parts of dichloromethane, 12.24 parts of terephthaloyl chloride, and 28.56 parts of isophthaloyl chloride is added dropwise to the reactor. . After reacting at a temperature of 10° C. for half an hour, 1.7 parts of tert-butylphenol was added, and the reaction was continued for 1 hour to stop the reaction. Neutralize the reaction solution with acetic acid until neutral, let it stand to separate the two phases, add the next layer of organic matter dropwise to methanol under vigorous stirring for precipitation, filter the precipitate and wash it with distilled water until there is no chloride ion, and finally put The prod...
Embodiment 2
[0044]Example 2: 890 parts of distilled water, 18 parts of sodium hydroxide, 36 parts of 2.2-bis(4-hydroxyphenyl) propane (bisphenol A), and 0.2 part of triethylbenzyl ammonium chloride were added to a stirring In a three-necked flask with a device and a thermometer, the water phase was formed after stirring and dissolving; the oil phase composed of 232 parts of dichloromethane, 28.56 parts of terephthaloyl chloride and 12.24 parts of isophthaloyl chloride was added dropwise to the reactor. After the reaction was carried out at 20°C for half an hour, 1 part of phenol was added, and the reaction was continued for 1.5 hours before the reaction was stopped. Neutralize the reaction solution with acetic acid until it is neutral, let it stand to separate the two phases, add the organic phase of the next layer dropwise to the vigorously stirred n-hexane for precipitation, filter the precipitate and wash it with distilled water until there is no chloride ion, and finally put The produ...
Embodiment 3
[0046] Example 3: 990 parts of distilled water, 23 parts of sodium hydroxide, 48 parts of 2.2-bis(4-hydroxyphenyl) propane (bisphenol A), 0.5 part of cetyltrimethylammonium bromide, were added to a stirring In a three-necked flask with a device and a thermometer, the water phase was formed after stirring and dissolving; the oil phase composed of 336 parts of chloroform, 34.2 parts of terephthaloyl chloride and 22.8 parts of isophthaloyl chloride was added dropwise to the reactor. After reacting at a temperature of 30° C. for half an hour, 2 parts of tert-butylphenol were added, and the reaction was continued for 2 hours before stopping the reaction. Neutralize the reaction liquid with acetic acid until it is neutral, let it stand to separate the two phases, add the organic phase of the next layer dropwise to hot water under vigorous stirring for precipitation, filter the precipitate and wash it with distilled water until there is no chloride ion, and finally The product was dr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Intrinsic viscosity | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com