Orally disintegrating tablet of coenzyme Q10 and preparation method thereof
An orally disintegrating tablet and coenzyme technology, which is applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, and pill delivery, etc., can solve problems such as the absence of orally disintegrating tablets of coenzyme Q10, accelerated degradation of coenzyme Q10, and low melting point of coenzyme Q10. , to achieve the effect of convenient taking and carrying, fast onset and short disintegration time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
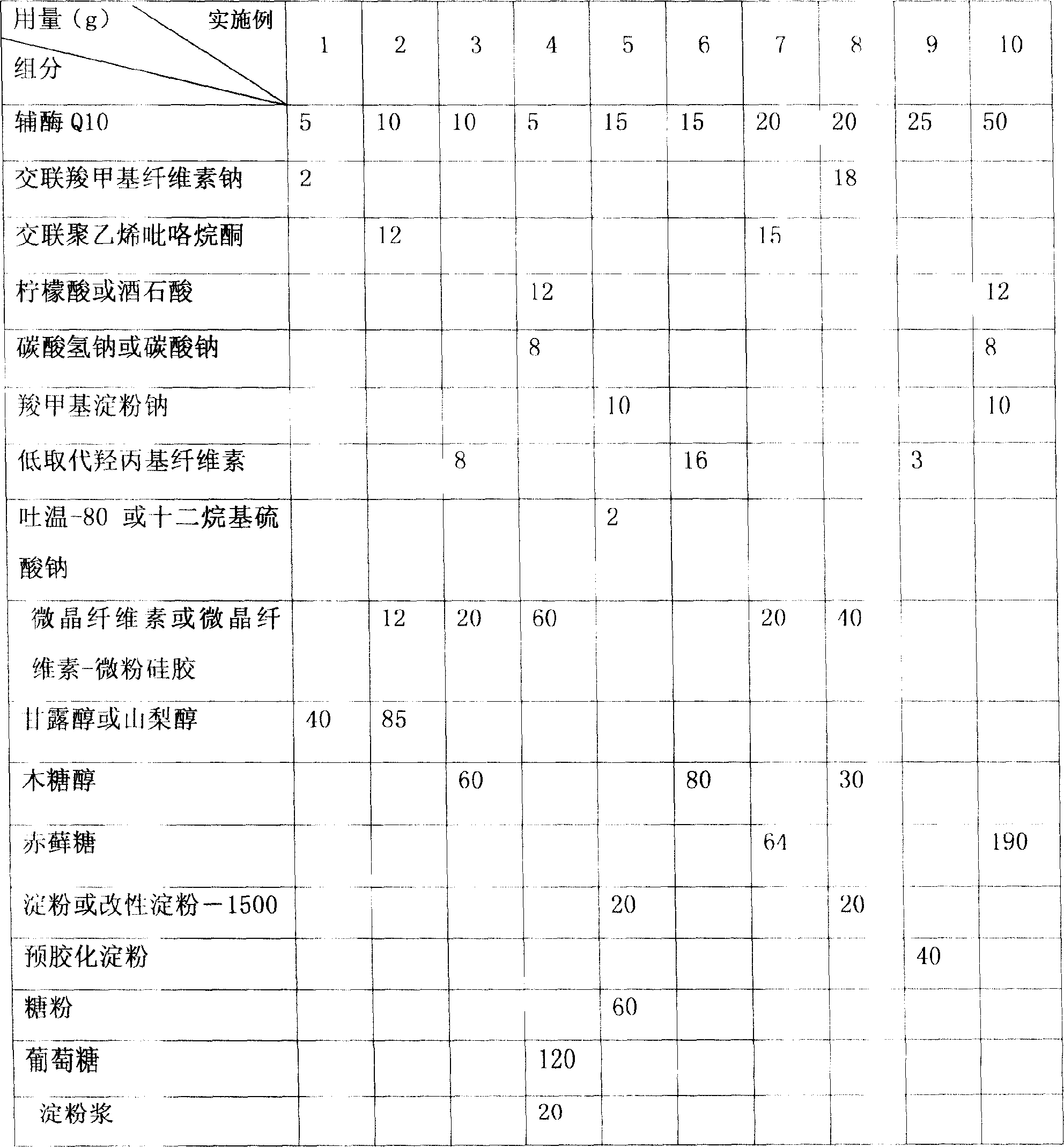
Examples
preparation example Construction
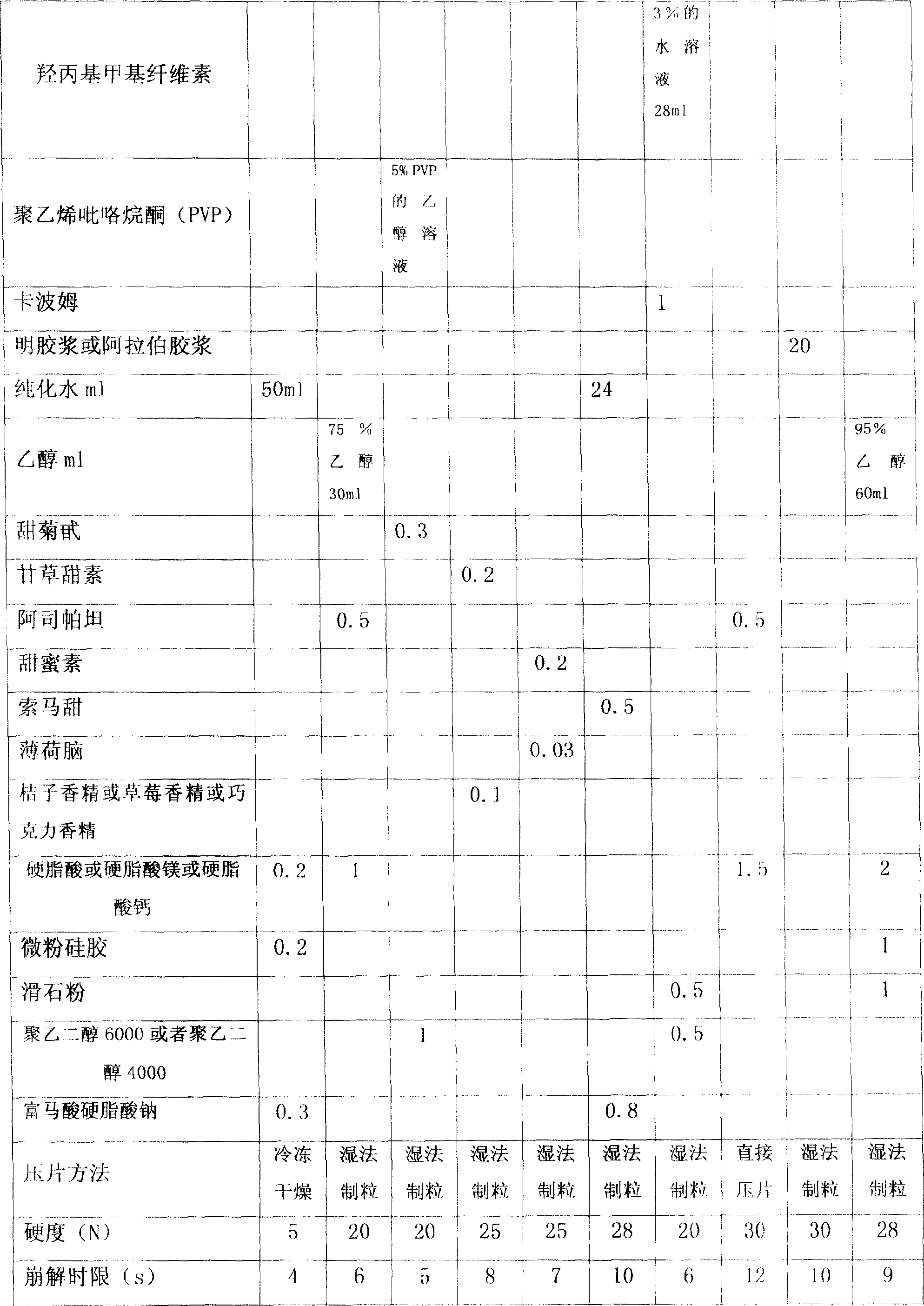
[0054]In the preparation method of the above-mentioned coenzyme Q10 orally disintegrating tablet, the tablet is pressed at a temperature of 20°C to 25°C and a relative humidity of 50% to 60%, and the speed of the tablet press is controlled at 20 rpm to 60 rpm It can effectively solve the problem of sticking and punching, so that the coenzyme Q10 orally disintegrating tablet can be industrialized and mass-produced smoothly.
[0055] In the present invention, the coenzyme Q10 can also be prepared into a solid dispersion, clathrate or microcapsule, and then prepared into an orally disintegrating tablet. In order to better illustrate the preparation method of the coenzyme Q10 orally disintegrating tablet, Example 11 is given as follows.
Embodiment 11
[0057] 1. Prescription:
[0058] Raw material - Coenzyme Q10 20g;
[0059] Capsule material-Eudragit E100 15g;
[0060] Bulking agent - mannitol 49g;
[0061] Disintegrant - low-substituted hydroxypropylmethylcellulose (L-HPC) 4g, croscarmellose sodium (CCNa) 3g;
[0062] Flavoring agent - stevioside 0.5g, orange essence 0.5g;
[0063] Lubricant-magnesium stearate 0.5g;
[0064] The total weight is 100g, and a total of 1000 tablets are made, 100mg / tablet, containing 20mg of coenzyme Q10.
[0065] 2. Preparation method
[0066] Step 1: Take Eudragit E100 is made into a certain concentration solution with absolute ethanol, and it is used as a capsule material solution for later use;
[0067] Step 2: Take coenzyme Q10 and suspend it with vertical airflow to prepare microcapsules by air suspension method. The obtained coenzyme Q10 microcapsules are dried, passed through a 80-mesh sieve, and set aside;
[0068] Step 3: Mix mannitol, L-HPC, CCNa, stevioside and orange e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com