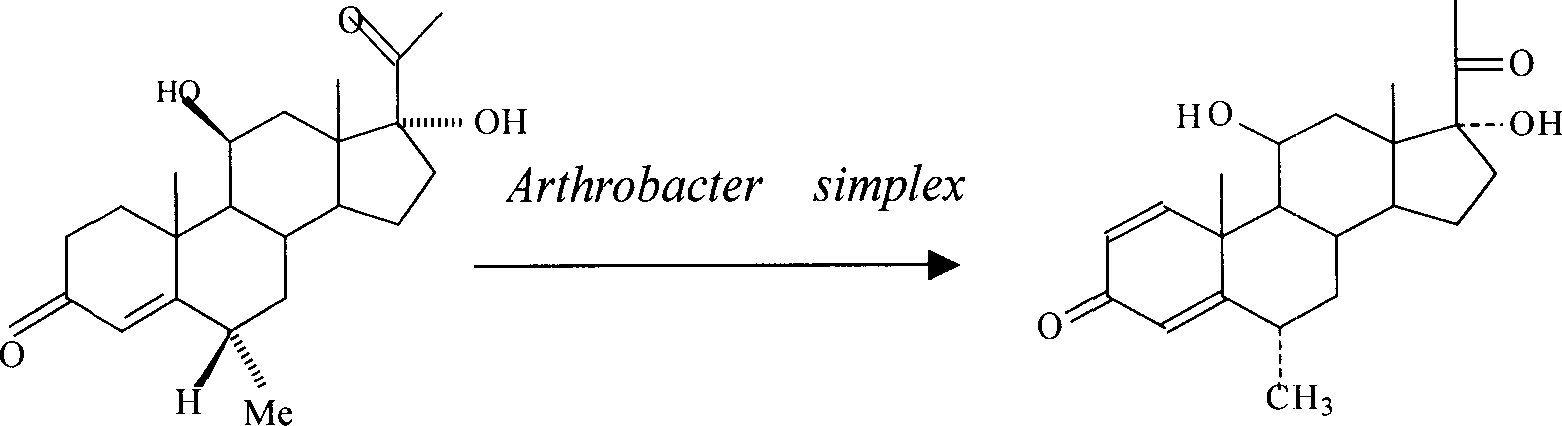
Process for preparing 6 alpha-methyl hydroprednisone
A methyl and seed technology, applied in the field of biotransformation, can solve the problems of affecting product quality and yield, difficult to treat waste water, and many side reactions, and achieve the effects of low cost, good selectivity and easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
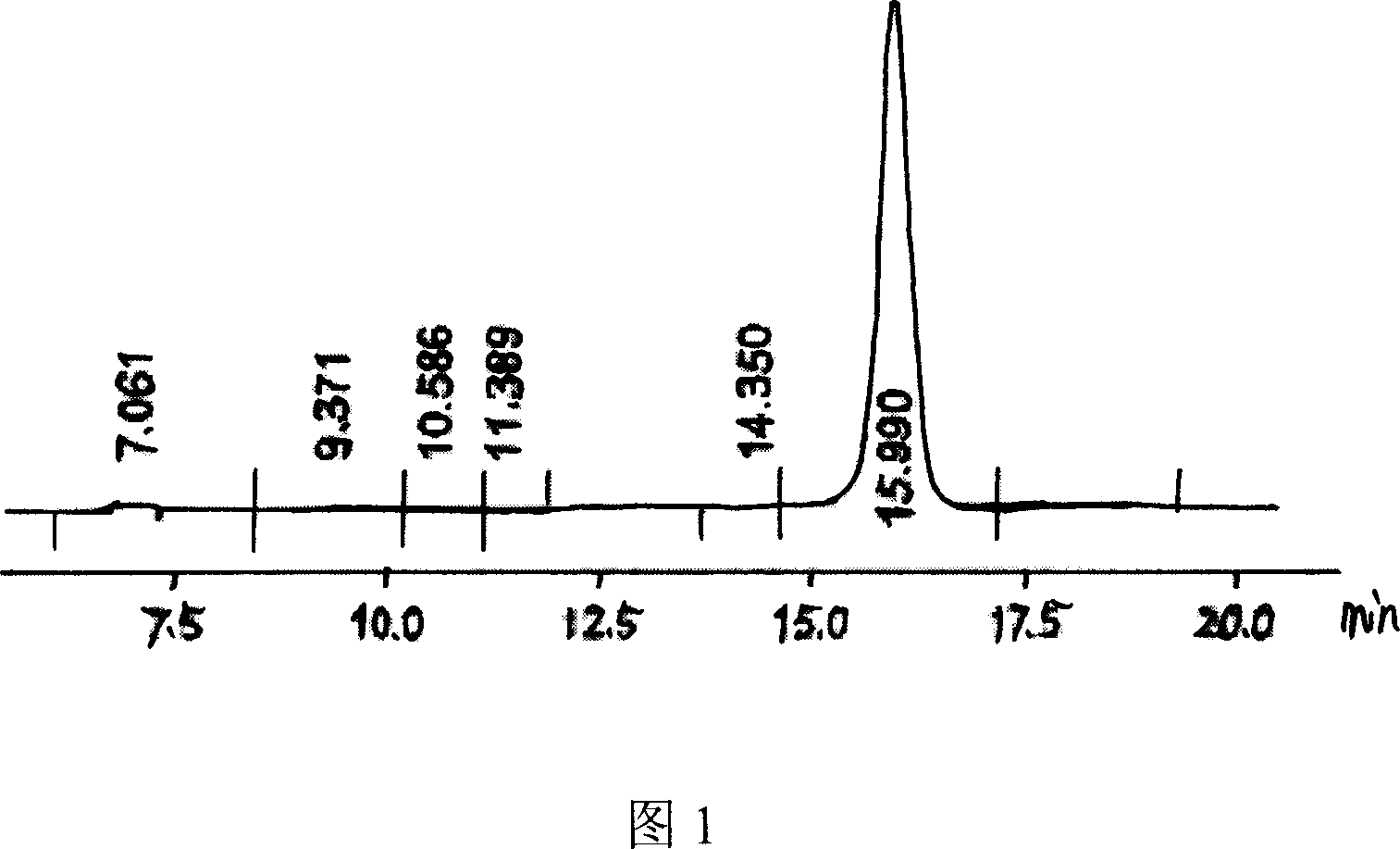
[0028] Example 1: Preparation of 1,4-diene-6α-methyl-3,20-diketone-11β,17α-dihydroxypregna
[0029] The formula of the slant medium is: 1.0% glucose; 1.0% yeast extract; 2.0% agar; pH7.0-7.2, sterilized at 121°C for 30min.
[0030] The seed medium and the fermentation medium have the same formula, consisting of: glucose 0.8%; corn steep liquor 1.0%; peptone 0.5%; KH 2 PO 4 0.20%; pH7.0~7.2, sterilized at 121℃ for 30min.
[0031] Pick a ring full of Arthrobacter simplex lawn from the mature slant, inoculate it into a 250ml seed bottle containing 50ml seed culture medium after sterilization, put it on a rotary shaker at 160r / min, and cultivate it at 28°C for about 10 ~16h, after sampling, the pH value of the seed solution is 6.7, OD 620 Value 0.298. Transfer 5ml of this seed solution to a 250ml fermentation bottle containing 50ml of fermentation medium, place it on a rotary shaker at 180r / min, and incubate at 28°C for about 12-20h, measure the pH value of the bacterial solu...
example 2~9
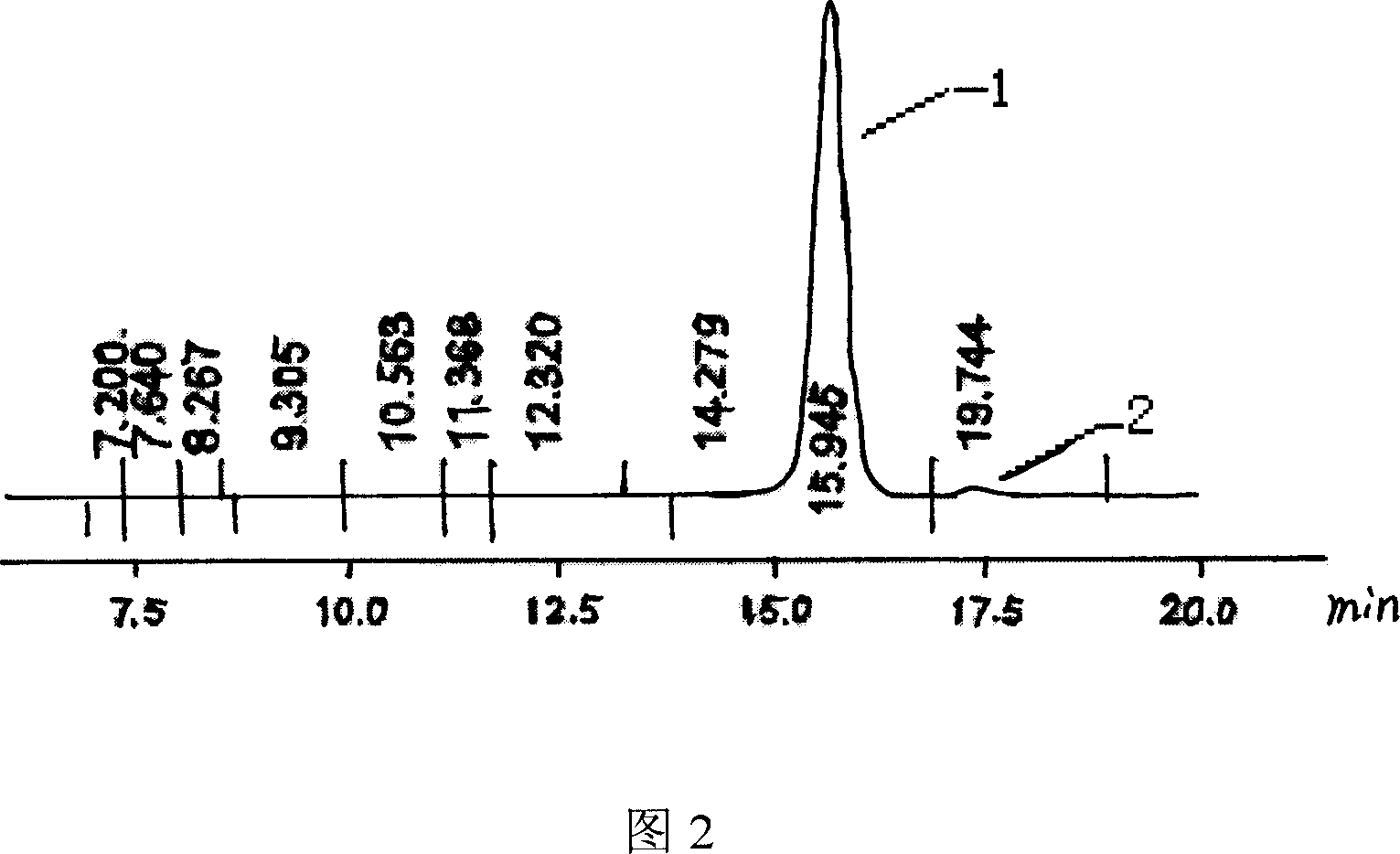
[0034] Examples 2-9: Preparation of 1,4-diene-6α-methyl-3,20-diketone-11β,17α-dihydroxypregna
[0035] With reference to the method of Example 1, change feeding intake 6α-methyl hydrocortisone substrate, pH value and OD value, the results are shown in Table 1:
[0036] Reality
example 10~15
[0037] Examples 10-15: Preparation of 1,4-diene-6α-methyl-3,20-dione-11β,17α-dihydroxypregna
[0038] With reference to the method of Example 1, change feeding concentration and vitamin K 3 The results are shown in Table 2:
[0039] Reality
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com