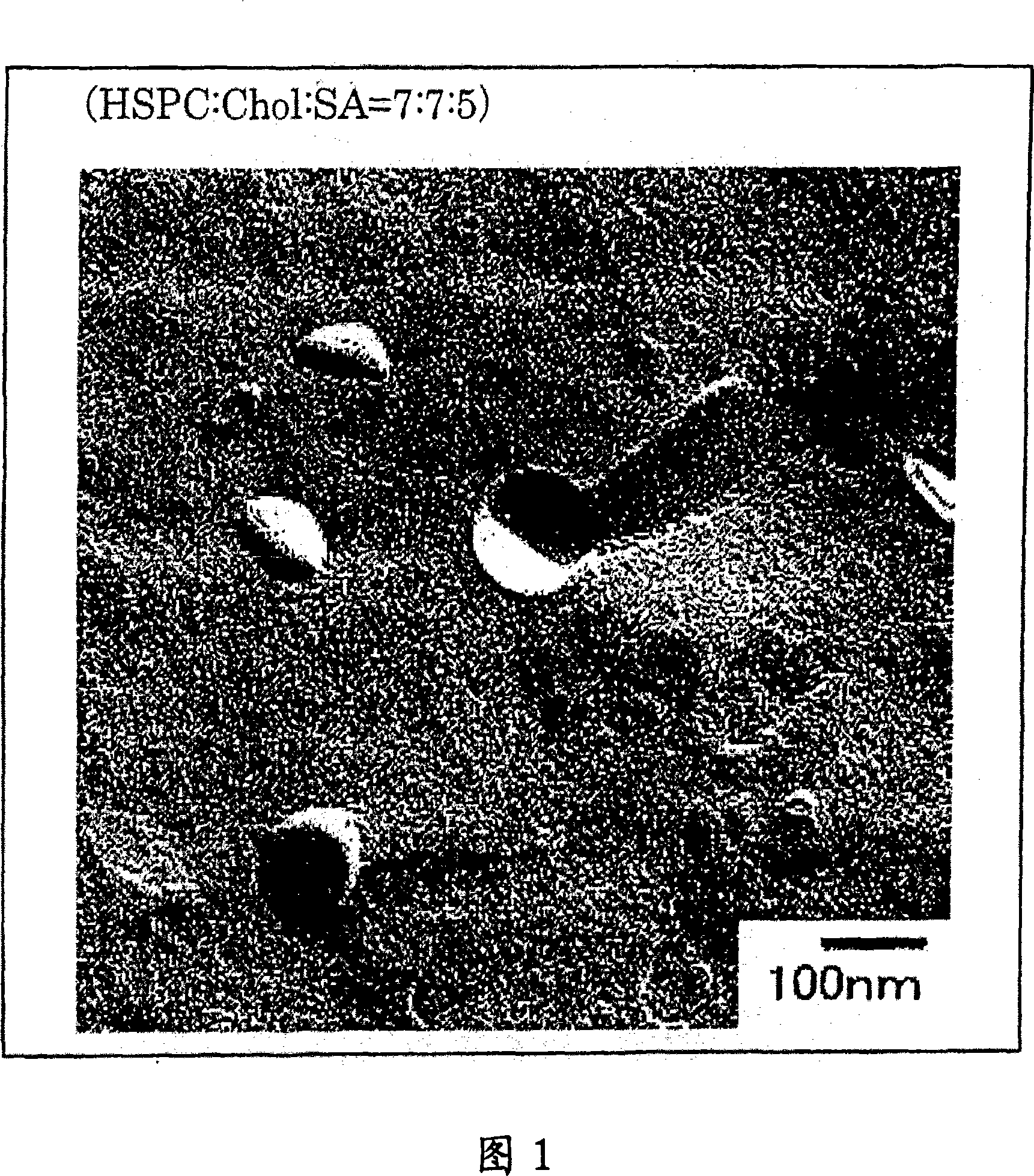
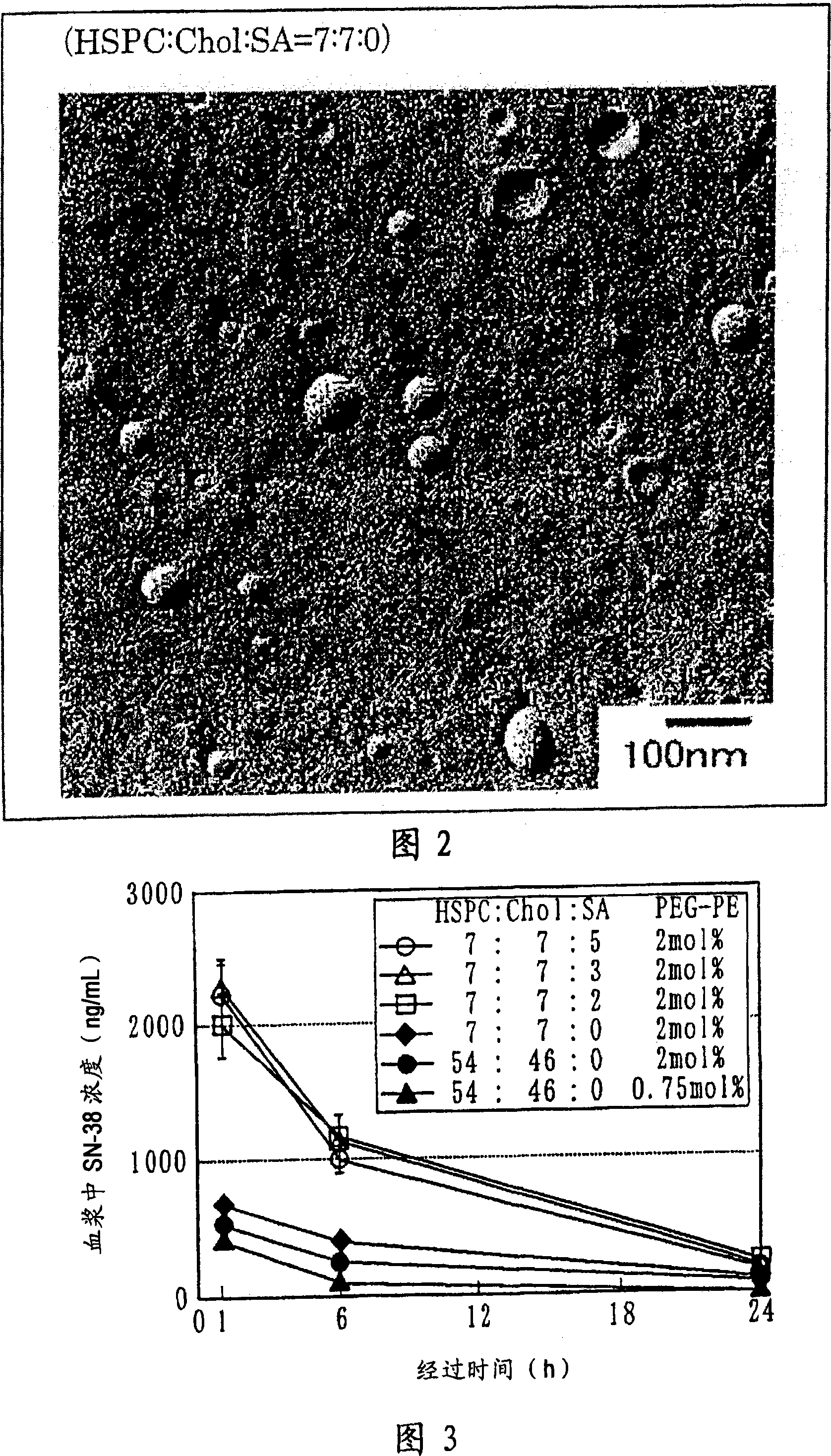
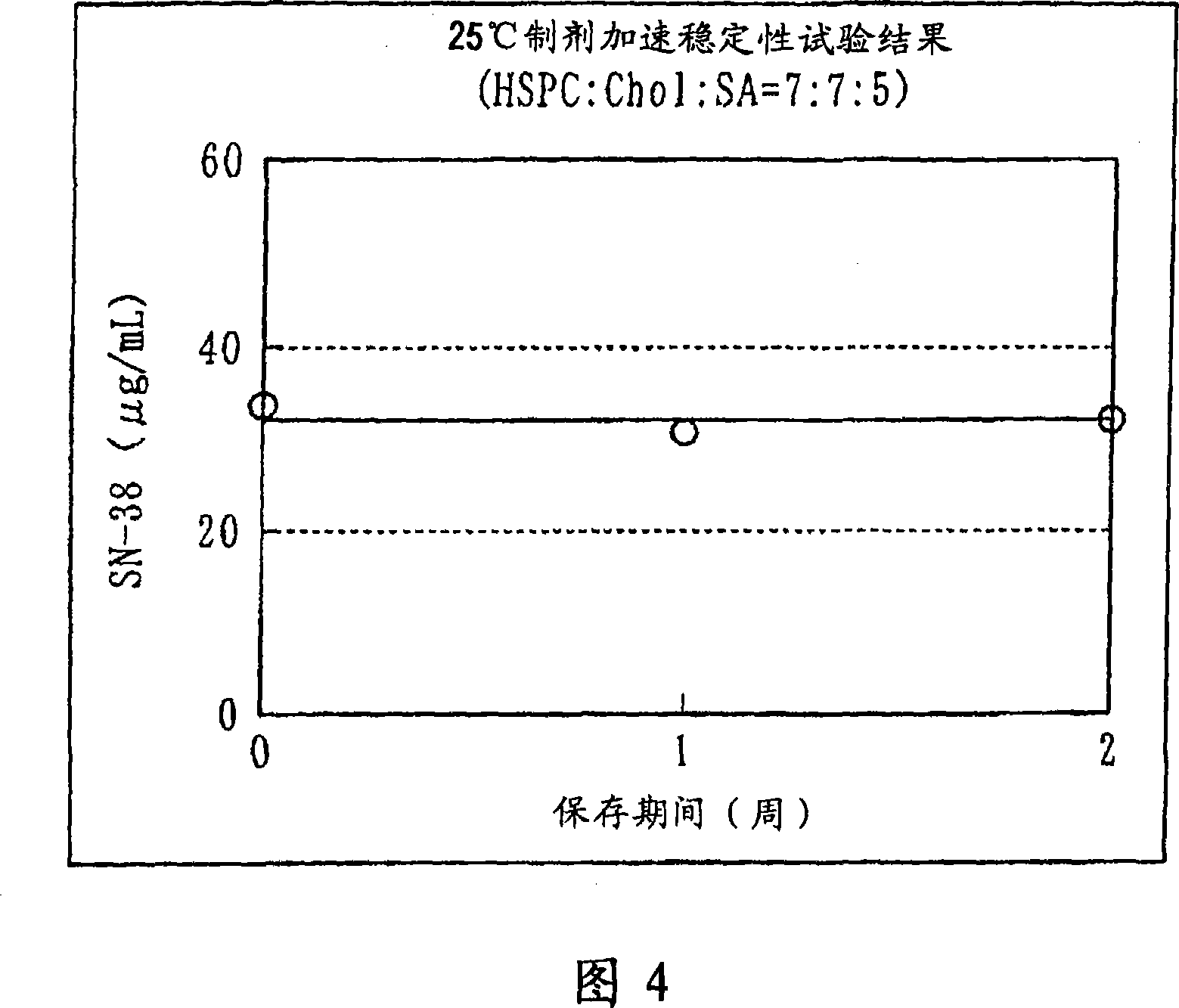
Liposome preparation containing slightly water-soluble camptothecin
A technology of liposome preparations and poor water solubility, which is applied in the field of liposome preparations, can solve problems such as difficulty in maintaining the concentration of SN-38, achieve excellent blood retention, excellent drug sustained release, and maintain the effect of drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~3
[0130] (1) Preparation of mixed materials
[0131] According to the specified molar ratio shown in Table 1, weigh hydrogenated soybean phosphatidylcholine (Lipoid company) (hereinafter referred to as HSPC), cholesterol (Solvay company) (hereinafter referred to as Chol), stearic acid (NOF Corporation) ( Hereinafter referred to as SA) and 7-ethyl-10-hydroxycamptothecin (SN-38, yakult head office) (hereinafter referred to as SN-38), which are dissolved in 200ml of tert-butanol heated to 60°C (Kanto Chemical ), cooled in an ice bath, and freeze-dried.
[0132] (2) scattered
[0133] 200 ml of physiological saline was added to the dried product obtained above, heated at 68° C., and pre-dispersed using Clearmix single (manufactured by M Technique Co.), the particle size was controlled using a microfluidizer (Microfluidics Co., Ltd.).
[0134] (3) Import of PEG-PE
[0135] Add 11.32mL of 36.74mg / mL physiological saline solution of polyethylene glycol (molecular weight 5000)-phosph...
Embodiment 4
[0162] As another mode of embodiment 3, the operation of embodiment 3 is repeated. The weighed value, the membrane composition of the obtained SN-38 liposome preparation, the drug load (medicine / lipid molar ratio, drug concentration), and the particle size are shown in Table 2.
[0163] [Table 2]
[0164] Table 2
[0165] Weighing value / g
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com