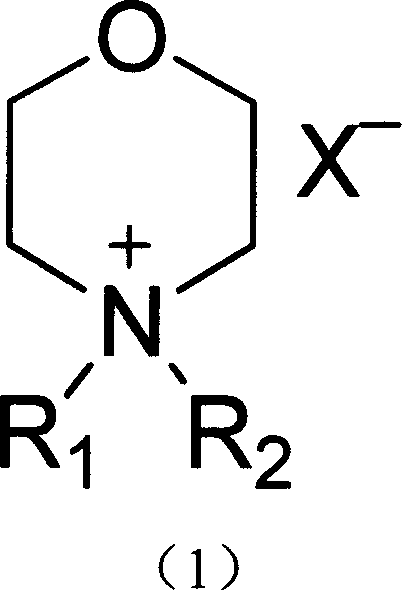Compound containing morpholine cation radical and its synthesis process and use
A technology of ionic groups and synthetic methods, applied in organic chemistry and other fields, can solve problems such as simple synthetic routes, high cost of raw materials, and restrictions on industrial production and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
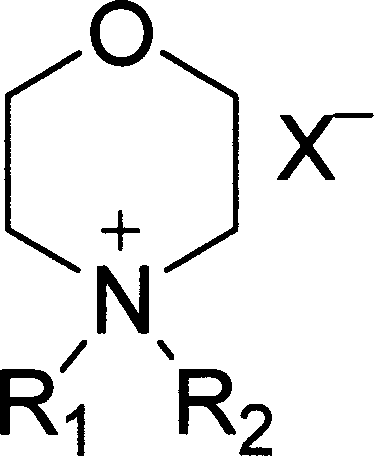
[0019] R in the above formula (1) 1 is methyl, R 2 is pentyl, X - The synthetic method that is the example compound of the present invention of fluoroborate anion comprises the following steps:
[0020] a. Add n-pentane bromide to N-methyl-morpholine (the molar ratio of N-methyl-morpholine and n-pentane bromide is 1: 1.05~6), the solvent can be methanol, ethanol, propanol , isopropanol, N,N-dimethylformamide, etc., react by heating, the reaction temperature is 80°C, the pressure is 1-10 atmospheres, and the reaction time is 10-200 hours.
[0021] b, after the completion of the reaction (chromatographic detection), add ammonium fluoroborate (the molar ratio of N-pentyl N-methyl-morpholine ammonium bromide to ammonium fluoroborate is 1: 1.05~6) in the above reaction solution, and the reaction temperature The temperature is 30°C, and the reaction time is 10-200 hours. The reaction product was purified to obtain N-pentyl N-methyl-morpholine ammonium fluoroborate.
[0022] Simil...
Embodiment 1
[0025] Preparation of N-isobutyl-N-methyl-morpholine ammonium chloride
[0026] With 101 grams of N-methyl-morpholine, 180 grams of chloroisobutane and 150 grams of solvent ethanol in the reaction device, the reaction temperature is 80 ° C, the pressure is 1 atmosphere, and the reaction time is 10 hours. After the reaction ( chromatographic detection), the solvent was evaporated. The reaction product is separated by a chromatographic column equipped with 20-200 times its weight of silica gel, and the mixture of cyclohexane and acetone is used as the eluent. After purification, N-isobutyl-N-methyl-morpholine chloride can be obtained Ammonium compound, stable to air, easy to absorb water, yield 94%.
[0027] m.p.: 36°C
[0028] High resolution mass spectrometry: [M-Cl] + =157
[0029] 1 HNMR (D 2 O, δ / ppm relative to TMS): 3.852-3.866(t, 5H), 3.415-3.418(m, 4H), 3.332(s, 3H), 1.714(m, 3H), 1.332(t, 3H), 0.964 (t, 3H).
Embodiment 2
[0031] Preparation of N-octyl-N-ethyl-morpholine ammonium bromide
[0032] With 101 grams of N-ethyl-morpholine, 200 grams of n-octane bromide and 150 grams of solvent acetonitrile in the reaction device, the reaction temperature is 100 ° C, the pressure is 4 atmospheres, and the reaction time is 80 hours. After the reaction is completed ( chromatographic detection), the solvent was evaporated. The reaction product is separated several times with a chromatographic column filled with 40 times its weight of alumina, using a mixture of dichloromethane and ethanol as the eluent, collecting the eluate, and concentrating to obtain N-octyl-N-ethyl -A morpholine ammonium bromide compound, stable to air, easy to absorb water, with a yield of 93%.
[0033] m.p.: 50°C
[0034] High resolution mass spectrometry: [M-Br] + =228
[0035] 1 HNMR (D 2 O, δ / ppm relative to TMS): 3.818(t, 4H), 3.425(t, 6H), 3.281(t, 2H), 1.734(t, 2H), 1.251(t, 13H), 0.961(t, 3H )
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com