Polycationic compounds and uses thereof
A polycation, compound technology, applied in 92,744 and 5,202,352. , another aspect involves the regulation of domains in animals or humans in need of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0256] A syrup or suspension is prepared by adding the active compound to a concentrated, aqueous solution of a sugar (eg, sucrose) along with any auxiliaries. Such adjuvants may include flavourings, sugar crystallization retarding agents or agents which increase the solubility of any other ingredient, for example polyols such as glycerol or sorbitol.
[0257] Formulations for rectal administration may be presented as suppositories, with conventional carriers such as cocoa butter or Witepsol S55 (trade name of Dynamite Nobel Chemical, Germany) used as suppository base.
[0258] Alternatively, the compounds can be administered as liposomes or microspheres (or microparticles). Methods for preparing liposomes and microspheres for administration to patients are well known to those skilled in the art. US Patent No. 4,789,734 describes methods for encapsulating biological material into liposomes, the contents of which are incorporated herein by reference. In general, the material ...
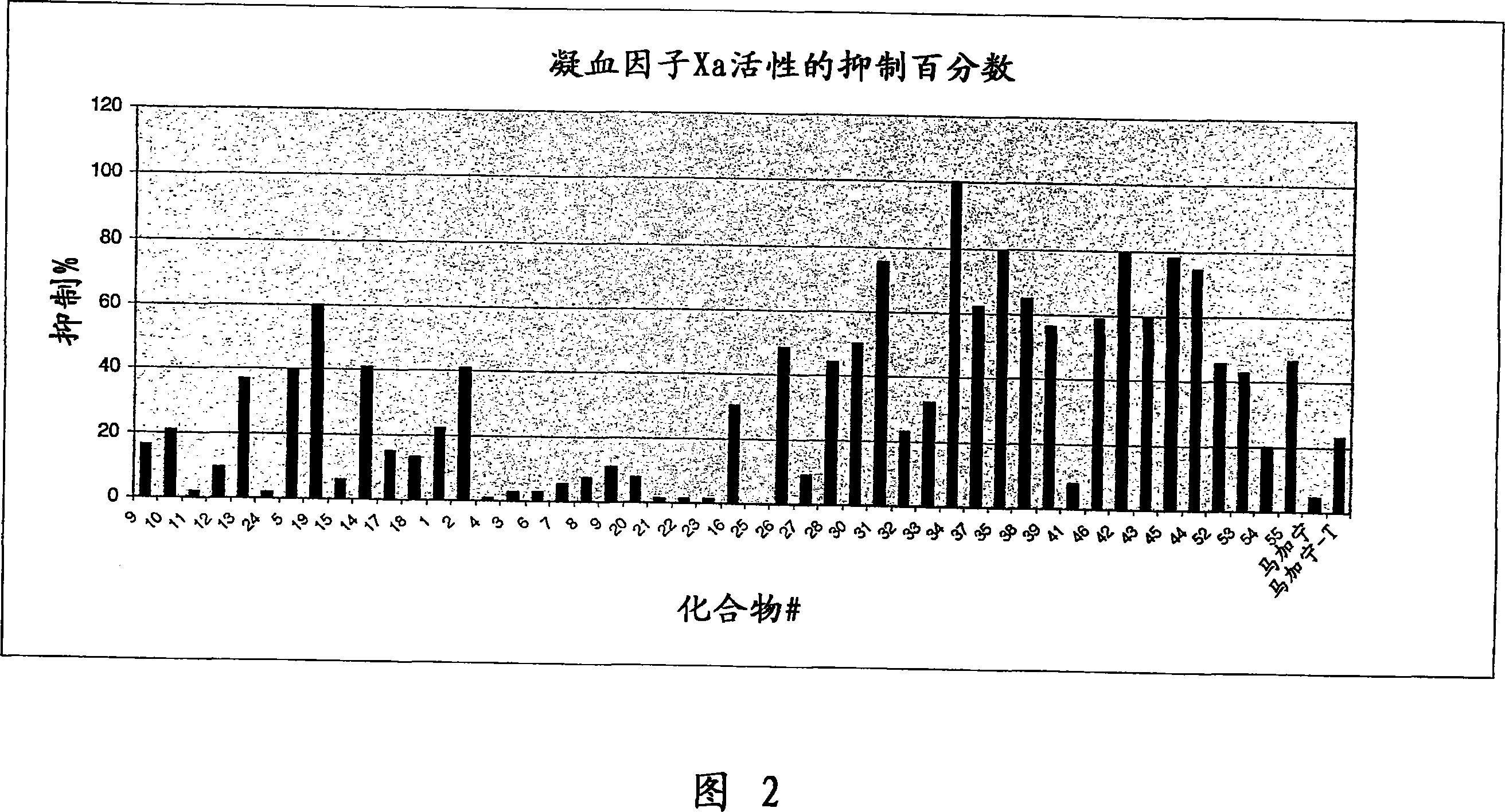
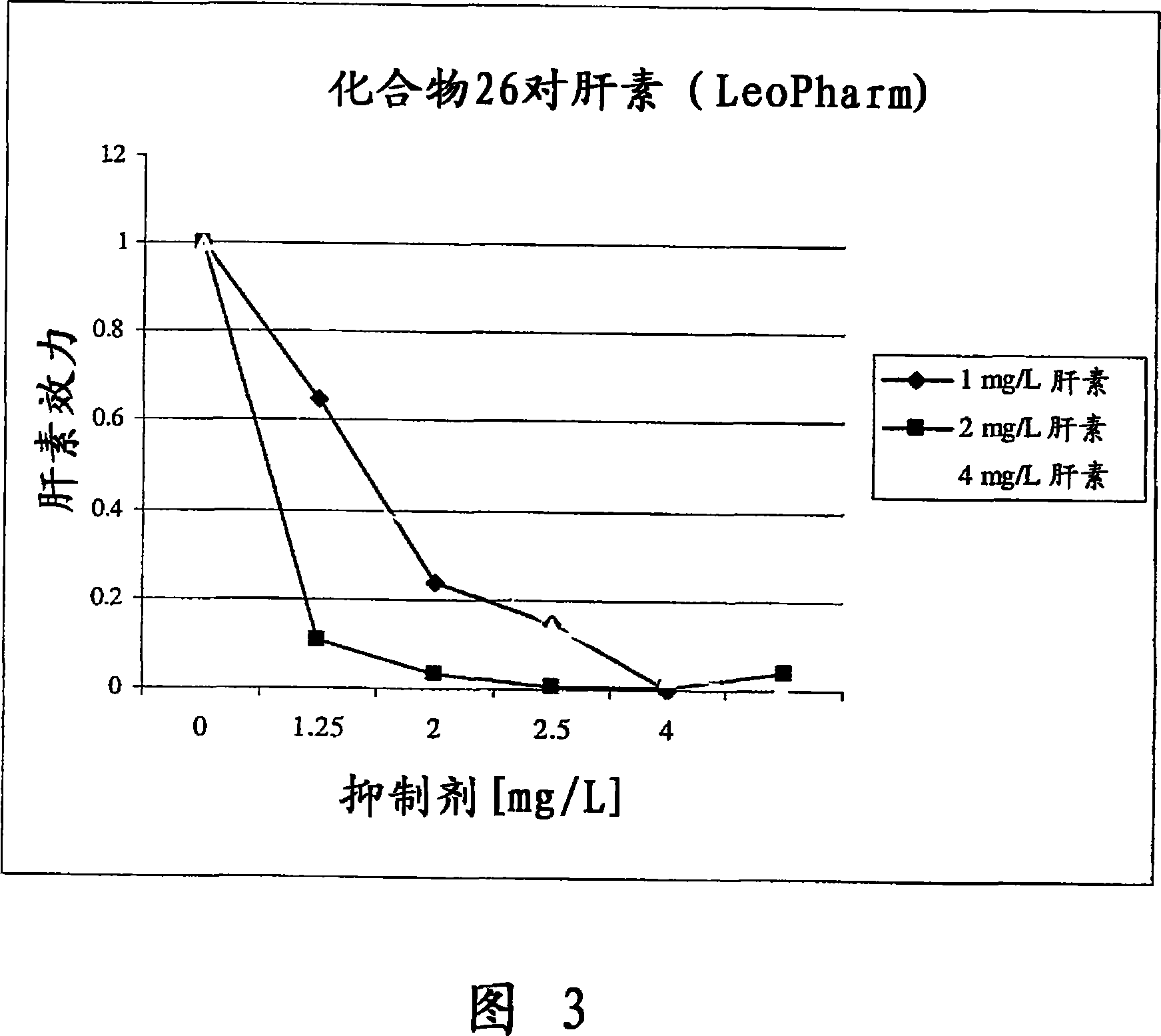
Embodiment 1
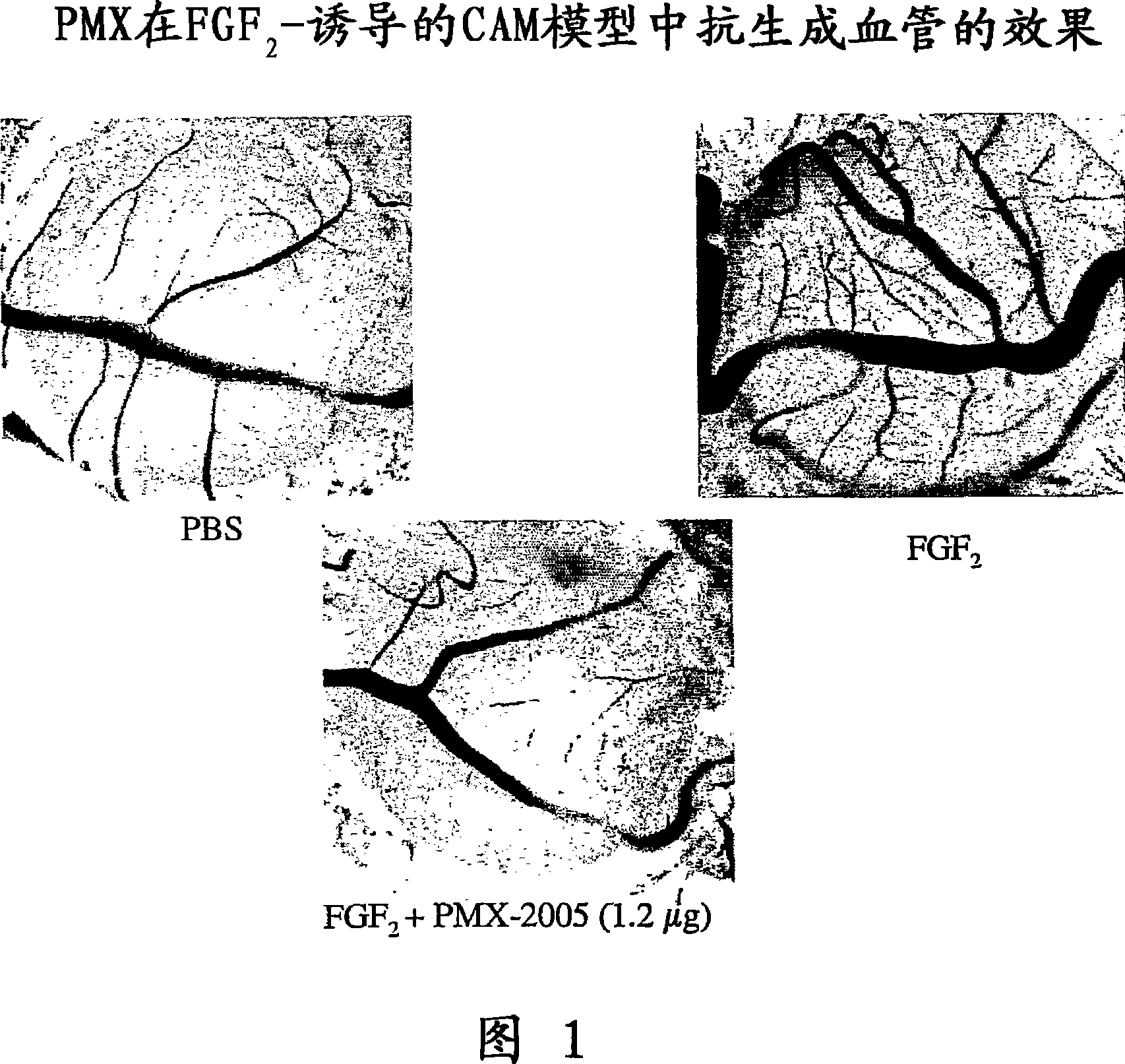
[0263] The following examples illustrate the anti-angiogenic effect of exemplary polycationic compounds of the invention. Ten-day-old embryos purchased from Spafas, Inc. (Preston, CT) were incubated at 37°C and 55% relative humidity. With the aid of a light-transmitting inspection light in the dark, a hypodermic needle is inserted into the casing to form a small hole to conceal the air cell. When viewed under light transmission, a second hole is formed on the egg's wider side piercing the shell, which passes directly through the avascular portion of the embryo's membrane. A pseudo-air sac is created under the second hole by applying negative pressure to the first hole, which separates the chorioallantoic membrane (CAM) from the shell. Use a small craft wheel (Dremel, Division of Emerson Electric Company Racine, Wisconsin) to cut out approximately 1.0 cm past the dislodged CAM on the shell 2 The fenestration allows direct access to the CAM below. Discs of #1 filter paper (Wh...
Embodiment 2
[0277] The following examples illustrate the inhibition of endothelial cell tube formation by polycationic compounds of the invention. Differentiation of endothelial cells was detected using the method developed by Grant et al. (Grant et al., In Vitro Cell Dev. Biol., 27A: 327-336 (1991). Matrigel without phenol red Matrix (commercially available from Becton Dickinson, Bedford, MA) was thawed overnight at 4°C. Using a cold pipette tip, dispense 3.0 mg / well Matrigel Put the matrix into a cold 24-well plate. Allow Matrigel during 30 min incubation at 37 °C Matrix polymerization.
[0278] At 37°C, 5% CO 2 Human umbilical vein endothelial cells (HUVEC) were maintained in endothelial cell growth medium with 2% fetal calf serum (EGM) and 95% humidity. Tube assays were performed in endothelial basal medium (EBM), supplemented with 0.5% bovine serum albumin (BSA) and a 1:100 dilution of insulin-transferrin-selenium-G supplement (I-T-Se, 100X) . HUVECs were trypsinized, ce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com