Expression of proteins in milk
a technology of recombinant proteins and milk, which is applied in the direction of viruses/bacteriophages, peptide sources, etc., can solve the problems of high cost, unreliability, and high cost of recombinant production in prokaryotic cells, and achieves low cost and high level production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0022] Bovine Alpha S-1 Casein
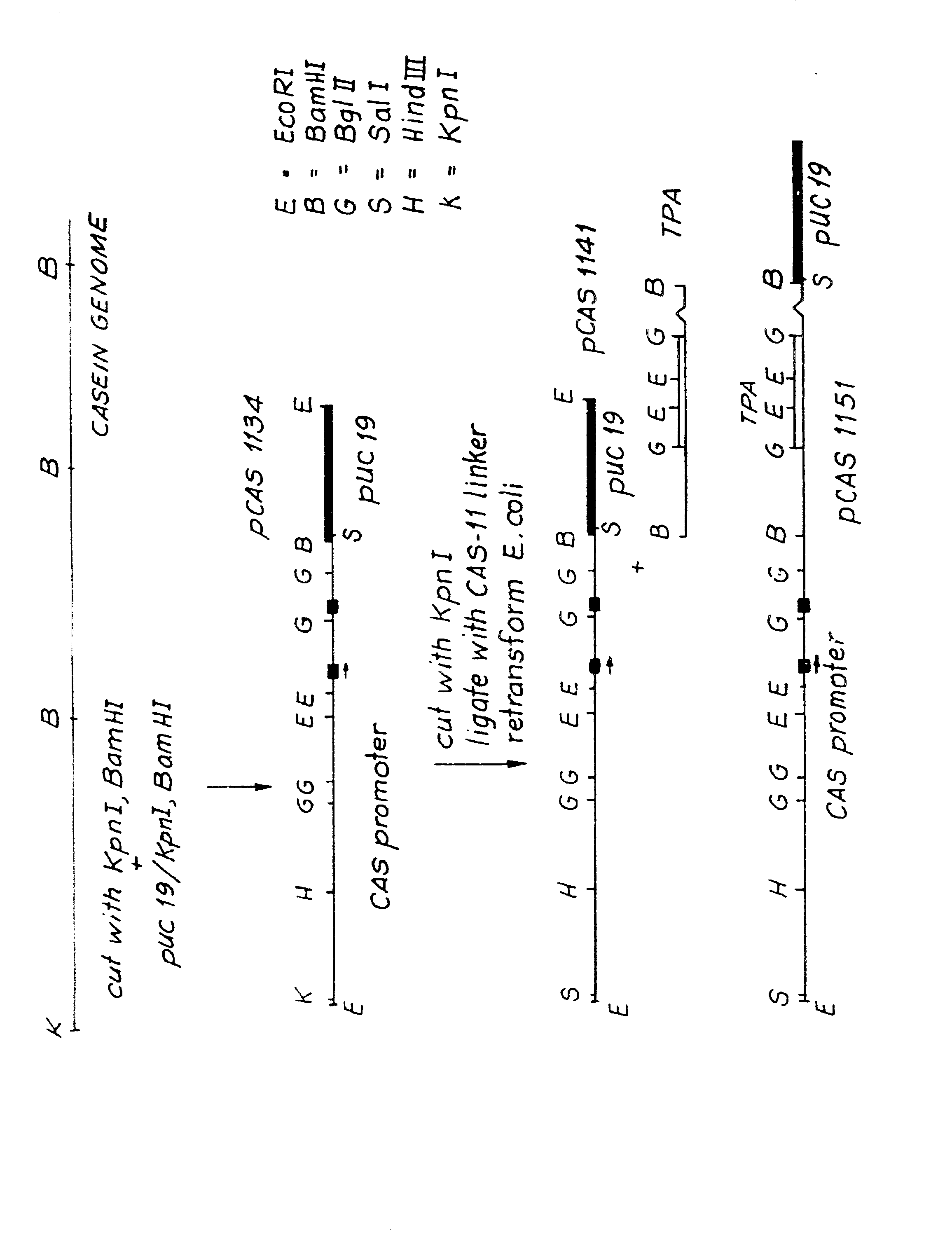
[0023] We cloned bovine alpha S-1 casein with a cosmid library of calf thymus DNA in the cosmid vector HC79 (from Boehringer Mannheim) as described by B. Hohn and J. Collins, Gene, 11, pp. 291-98 (1980). The thymus was obtained from a slaughterhouse and the DNA isolated by standard techniques well known in the art (T. Maniatis et al., "Molecular Cloning: A Laboratory Manual", Cold Spring Harbor Laboratory at page 271 (1982)). We isolated the cosmid library using standard techniques (F. Grosveld et al., Gene, 13, pp. 227-31 (1981)). We partially digested the calf thymus DNA with Sau3A (New England Bio Labs) and ran it on a salt gradient to enrich for 30 to 40 kb fragments. The partially digested DNA fragments were then ligated with BamHI digested HC79 cosmid vector, followed by in vitro packaging by lambda extracts (Amersham) following the manufacturer's recommendation. The in vitro packaged material was then used to infect the E.coli K-12 strain HB101 f...
example 2
Construction of the Cas-recombinant Product Construct
[0030] One recombinant protein that can be produced by the process of this invention is tissue plasminogen activator or TPA. As demonstrated below, the casein signal peptide was used to direct secretion of TPA from the mammary glands of transgenic mice carrying a construct according to this invention. In this construct, the nucleotide sequence of the casein signal peptide was fused to the sequence of mature TPA by RNA processing. The sequence of TPA has been described in D. Pennica et al., Nature, 301, pp. 214-21 (1983). In the TPA gene, as in the CAS gene, there is a BamHI site in Intron II which separates the signal peptide from the mature sequence [R. Fisher et al., J. Biol. Chem., 260, pp. 11223-30 (1985)]. The cDNA of TPA shows the BglII site in Exon III at amino acid #3 of mature TPA.
[0031] We subcloned a 1.7 kb fragment from tha genomic clone of TPA [R. Fisher et al., supra] using BamHI-BglII. The 1.7 kb fragment contained ...
example 3
Transgenic Incorporation of the Construct into Mice
[0033] The procedure for transgenic incorporation of the desired genetic information into the developing mouse embryo is established in the art [B. Hogan et al., "Manipulating The Mouse Embryo: A Laboratory Manual" Cold Spring Harbor Laboratory (1986)]. We used an F1 generation (Sloan Kettering) cross between C57B1 and CB6 (Jackson Laboratories). Six week old females were superovulated by injection of Gestile (pregnant mare serum) followed by human chorionic gonadotropin two days later. The treated females were bred with C57B1 stud males 24 hours later. The preimplantation fertilized embryos were removed within 12 hours following mating for microinjection with DNA and implantation into pseudopregnant females.
[0034] We injected the construct by first digesting the cumulus cells surrounding the egg with Hyaluronidase. The construct was injected into the pronucleus of the embryo until it swelled 30% to 50% in size. We then implanted th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com