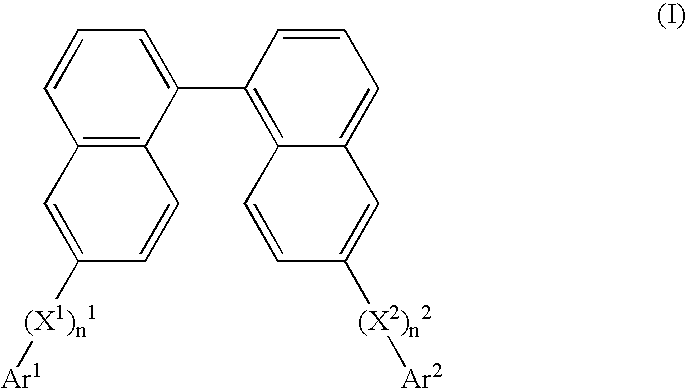
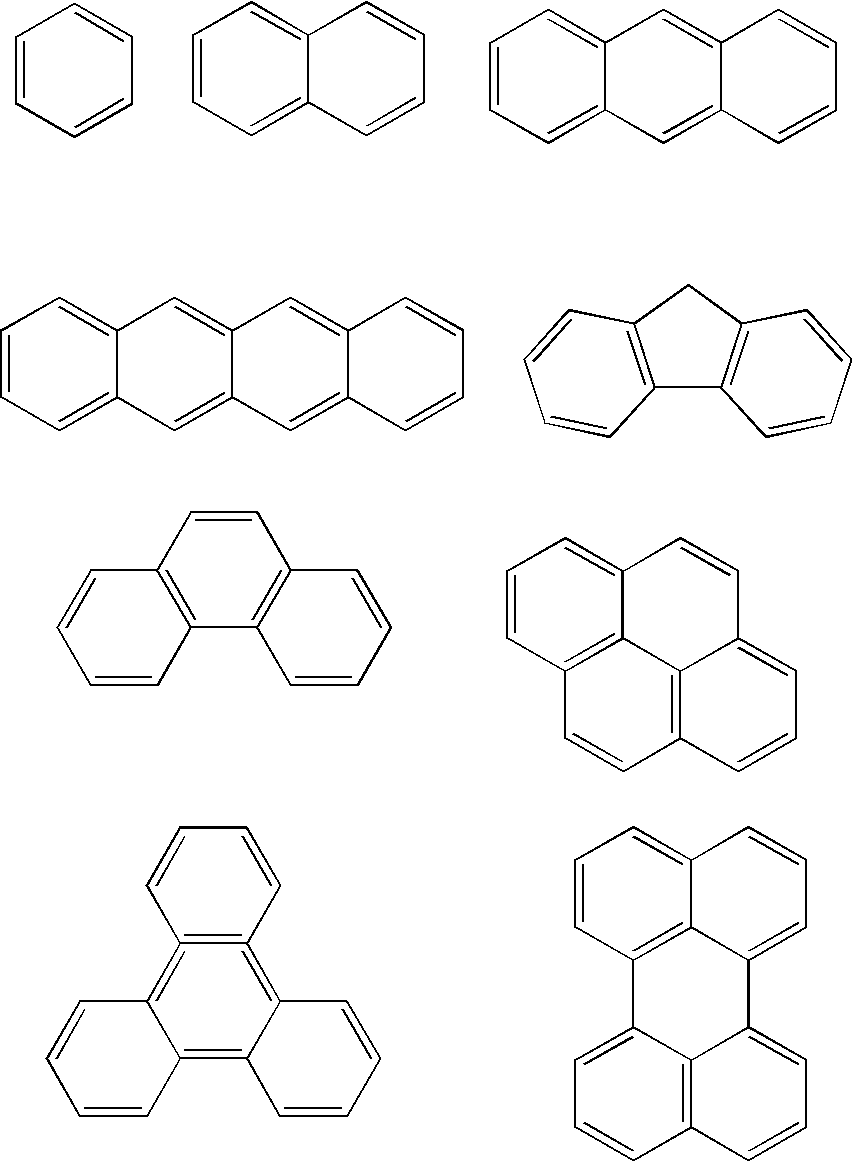
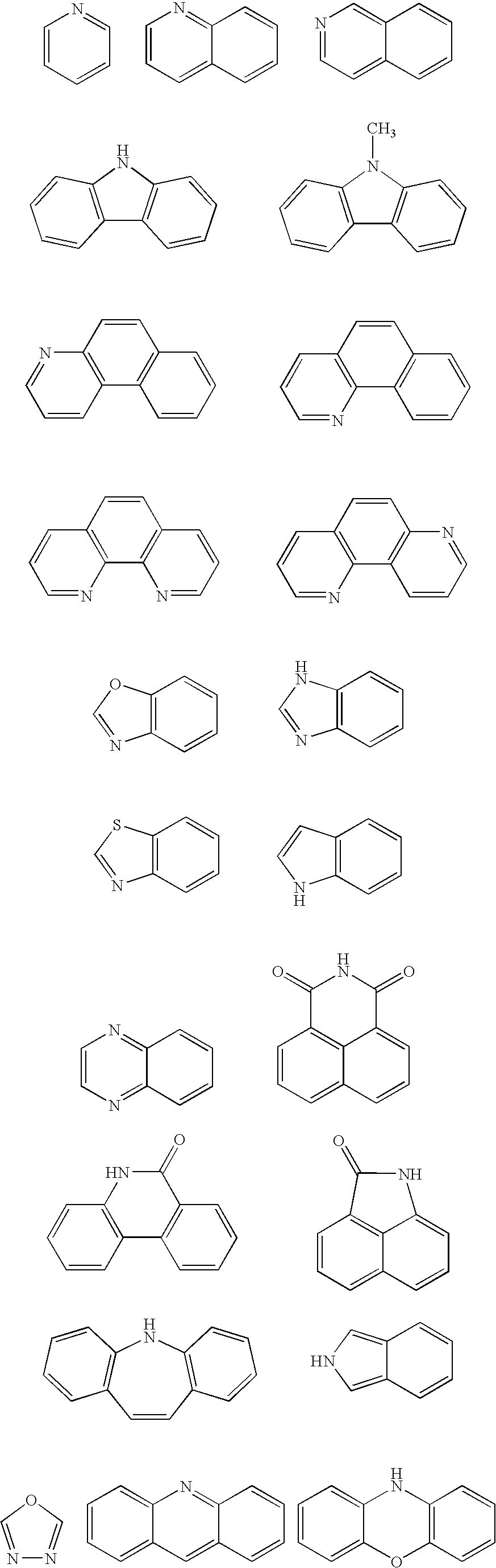
Binaphthol based chromophores for the fabrication of blue organic light emitting diodes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 2
[0081] This example describes the synthesis and characterization of a binaphtyl compound of the formula (IV). 22
[0082] A round bottom flask was charged with 6,6'dibromo-2,2'dilaexyloxy-1-,1'binaphthyl (895 mg, 1.46 mmol), 1-pyrene boronic ester (1.2 g, 3.66 mmol, 2.5 equivs), sodium bicarbonate (845.3 mg, 7.31 mmol and Pds(PPh.sub.3).sub.4 (84.5 mg, 0.073 mmol, 0.05 equivs). The mixture was evacuated and refilled with Argon three times and dissolved in Toluene / THF / water (3:3:1). The solution was degassed three times and the mixture heated under Argon at 86.degree. C. for 48 hrs. After heating, the solution was cooled down, the solvent evaporated, water was added and the aqueous phase was extracted with chloroform three times. The combined organic phases were washed with brine, dried, evaporated and concentrated under vacuum to afford the crude product. The resulting solid was purified by flash column chromatography on silica gel (hexanes 75%, chloroform 25%) to afford the compound o...
example 3
[0085] This example provides a characterization of absorption and emission in solution and thin films of the compound of formula (III) or the compound of formula (IV). The PL, and absorption in solution were measured from chloroform solutions (10.sup.-6 and 4.times.10.sup.-6 M respectively). The thin films were prepared by vacuum evaporation. The results obtained are summarized in FIG. 1. In FIG. 1A, the absorption of the compound of formula (III) is shown in chloroform solution 2 and in the solid state 4. In FIG. 1B, the normalized PL of the compound of formula (III) is shown in solution 6 and in thin film 8. In FIG. 1C, the absorption of the compound of formula (IV) is shown in chloroform solution 10 and in the solid state 12. In FIG. ID, the normalized PL of the compound of formula (IV) is shown in solution 14 and in thin film 16. The compound of formula (III) in solution gave a blue emission (.lambda..sub.PL at 430 nm) under UV excitation at 351 nm. The quantum efficiency was 0....
example 4
[0086] This example demonstrates the fabrication of electroluminescent devices using blue emitting binaphthol compounds.
[0087] The devices were fabricated by vacuum evaporation of the organic layers (CuPc / .alpha.-NPD / EML / SAlq / Alq.sub.3) on glass / ITO substrate at about 10.sup.-6 Torr in the same chamber without breaking vacuum. The ITO layer on glass substrate was 120 nm thick, and a stripe pattern with a 2-mm wide ITO layer was etched by photolithographic techniques. The patterned ITO glass was previously ultrasonically cleaned using a detergent, rinsed in water and finished with UV-ozone method. The rates of deposition of the organic layers was between 1.0 and 2.3 .ANG. / s and the temperatures of deposition were within a range of 206-304.degree. C., depending on the layer. The cathode was deposited in another chamber with a metal shadow mask that defines a 2-mm stripe cathode pattern perpendicular to the ITO anode stripe 20. FIG. 2 is a top view of the device showing the 2-mm wide a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Fluorescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com