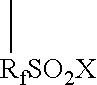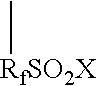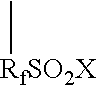Graft oligomeric electrolytes
a technology of oligomeric electrolytes and grafts, which is applied in the direction of non-aqueous electrolyte cells, cell components, electrochemical generators, etc., can solve the problems of insufficient electrochemical stability, insufficient charge/discharge rate capability of cells, and difficulty in processing limit their usefulness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Grafting of PSEPVE onto Dodecane
[0049] A 500 mL 3-neck round bottom flask, equipped with a condenser, magnetic stirring, addition funnel, and gas inlet, was charged under a nitrogen atmosphere with 100 grams of anhydrous dodecane (Aldrich), 80 grams of PSEPVE made according to Connolly et al., U.S. Pat. No. 3,282,875, and 4 grams of t-butyl peroxide (Aldrich). The mixture was heated in an oil bath to 125.degree. C. and stirred at this temperature for eight hours. The initially clear reaction mixture became cloudy as the reaction progressed. After eight hours, the mixture was allowed to cool to room temperature and was held overnight without stirring. Two clear layers were obtained. The top layer consisted mostly of unreacted dodecane and was decanted off. The bottom layer was transferred into a single-neck round bottom flask and was heated to 80.degree. C. in a Kugelrohr apparatus at 0.5 mm Hg to evaporate any unreacted materials. 75 g of a clear viscous liquid were obtained.
[0050] ...
example 2
Grafting of PSEPVE onto n-Heptane
[0051] A stainless steel 600 mL high pressure reactor (Parr Instrument Co., Moline, Ill.) was charged with 100 mL of n-heptane (Aldrich), 10 grams of PSEPVE, and 1 gram of t-butyl peroxide (Aldrich). The reactor was closed and then it was purged and vented three times with nitrogen. Stirring was started and the reactor was heated to 80.degree. C. After one hour the temperature was raised to 120.degree. C., and after four more hours to 130.degree. C. After one hour at 130.degree. C. the temperature was raised again to 140.degree. C. where it remained for the rest of the reaction time. Total reaction time was eight hours. After this time the reactor was cooled to room temperature where it remained overnight under nitrogen atmosphere. The reaction mixture was transferred to a 300 mL round bottom flask and the excess heptane was distilled off at atmospheric pressure. The remaining material was heated to 80.degree. C. in a Kugelrohr apparatus at 0.5 mm Hg...
example 3
Preparation of PSEPVE-Grafted Dodecane with Dicyanomethide Groups
[0053] A 500 mL 3-neck round bottom flask, equipped with a condenser, magnetic stirring, addition funnel, gas inlet, and under nitrogen atmosphere was charged with 25.7 grams of PSEPVE-grafted dodecane (containing an average of 2.8 PSEPVE grafts per molecule) prepared according to the method in Example 1, and 150 mL of anhydrous tetrahydrofuran (THF, Aldrich). After all the grafted dodecane dissolved, 1 gram of LiH (Aldrich) was added carefully in small portions. The reaction mixture was cooled to 0.degree. C. and a solution of 4.15 grams of malononitrile (Aldrich) in 50 mL of THF was added dropwise over a period of two hours. After the addition the mixture was allowed to warm up to room temperature where it remained stirring overnight under nitrogen. Methanol (20 mL) was added to destroy any traces of unreacted LiH and then the solvent was removed in a rotary evaporator. The viscous yellowish liquid obtained was disso...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com