Prenatal screening
a technology for prenatal screening and tissue, applied in the field of prenatal screening, can solve problems such as unworkable approaches to da
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Further Induction Optimization
[0239] This example describes additional experiments carried out to further determine the optimal induction time for an activating egg extract. Protein synthesis during the early part of the first cell cycle in activated eggs or egg extracts is required for preparation of an activating extract capable of efficient and complete genome replication. The required proteins can either be synthesized in intact eggs before preparation of extracts or in extracts including frozen / thawed extracts. As noted above, activating egg extract should be prepared from extracts induced for more than 10 minutes to enhance DNA synthesis activation activity.
[0240] It was found that proteins synthesized during the first 28-30 minutes in intact eggs (incubated at 20° C.) or during the first 60-80 minutes in a freshly prepared and activated extract (incubated at 25° C.), promote subsequent DNA replication. In contrast, proteins synthesized later in the first cell cycle, i.e., a...
example 2
cAMP Supplemented Activating Egg Extract
[0245] The affect of cAMP on DNA replication in activated Xenopus red blood cells was determined. Xenopus nuclei were isolated and pretreated by a method based on Coppock et al., Developmental Biology 131: 102 (1989), as follows: Xenopus blood was obtained from females by cardiac puncture and collected using a syringe half-filled with Barth's solution (88 mM NaCl, 2.3 mM KCl, 0.82 mM MgCl2 and 10 mM Hepes, pH 7.4) containing heparin (10 mg / ml); the blood was immediately diluted into 10 ml of ice-cold 0.6×SSC (1×SSC is 0.15 M NaCl, 0.015 M Sodium citrate, pH 7.0) containing 0.1 mg / ml heparin, 0.1 mM TPCK (N-tosyl-L-phenylalanine chloromethyl ketone), 0.1 mM TLCK (Na-p-Tosyl-L-lysine chloromethyl ketone), 0.05 mM PMSF (phenylmethylsulfonyl fluoride), 5 μg / ml leupeptin, and 31.25 mM Na2S2O5; bleeds containing clots, even small ones, were rejected; diluted blood was underlaid with 0.5 volumes of ice cold Metrizamide• (refractive index of 1.3660 i...
example 3
Caffeine Supplemented Activating Egg Extract
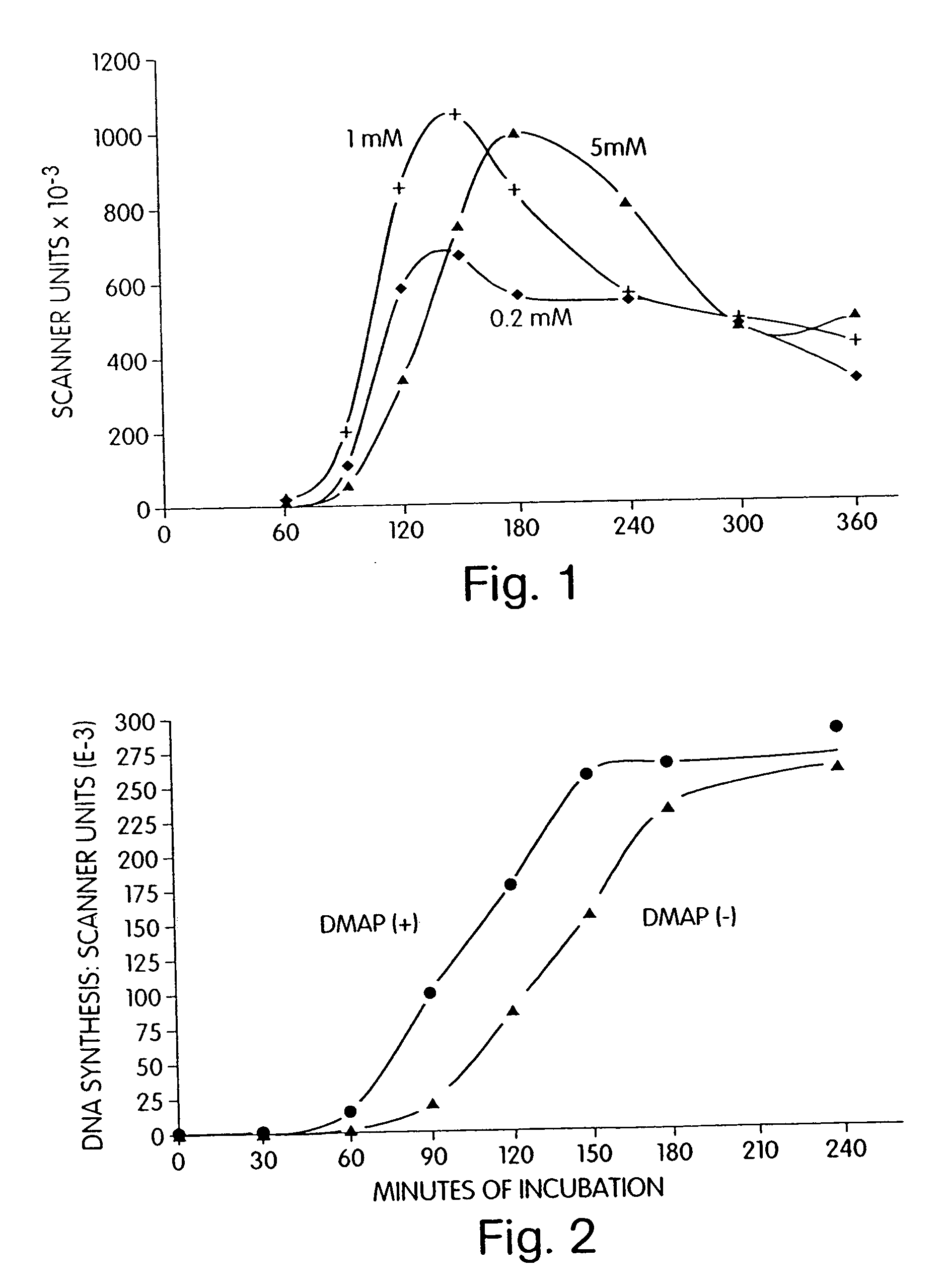
[0249] The effect of caffeine on DNA replication in activated Xenopus red blood cells was determined. The experimental conditions used were as described in Example 1 with the following changes: the concentration of cAMP was set at 0.3 μM and caffeine was added to the activating egg extract to a concentration of either 0.2 mM, 1.0 mM, or 5.0 mM.
[0250] As illustrated by FIG. 1, caffeine at 1.0 mM in the presence of 0.3 μM cAMP gave the highest initial rate and extent of DNA replication in activated nuclei. Thus, caffeine can increase the activation activity of an activating egg extract.
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com