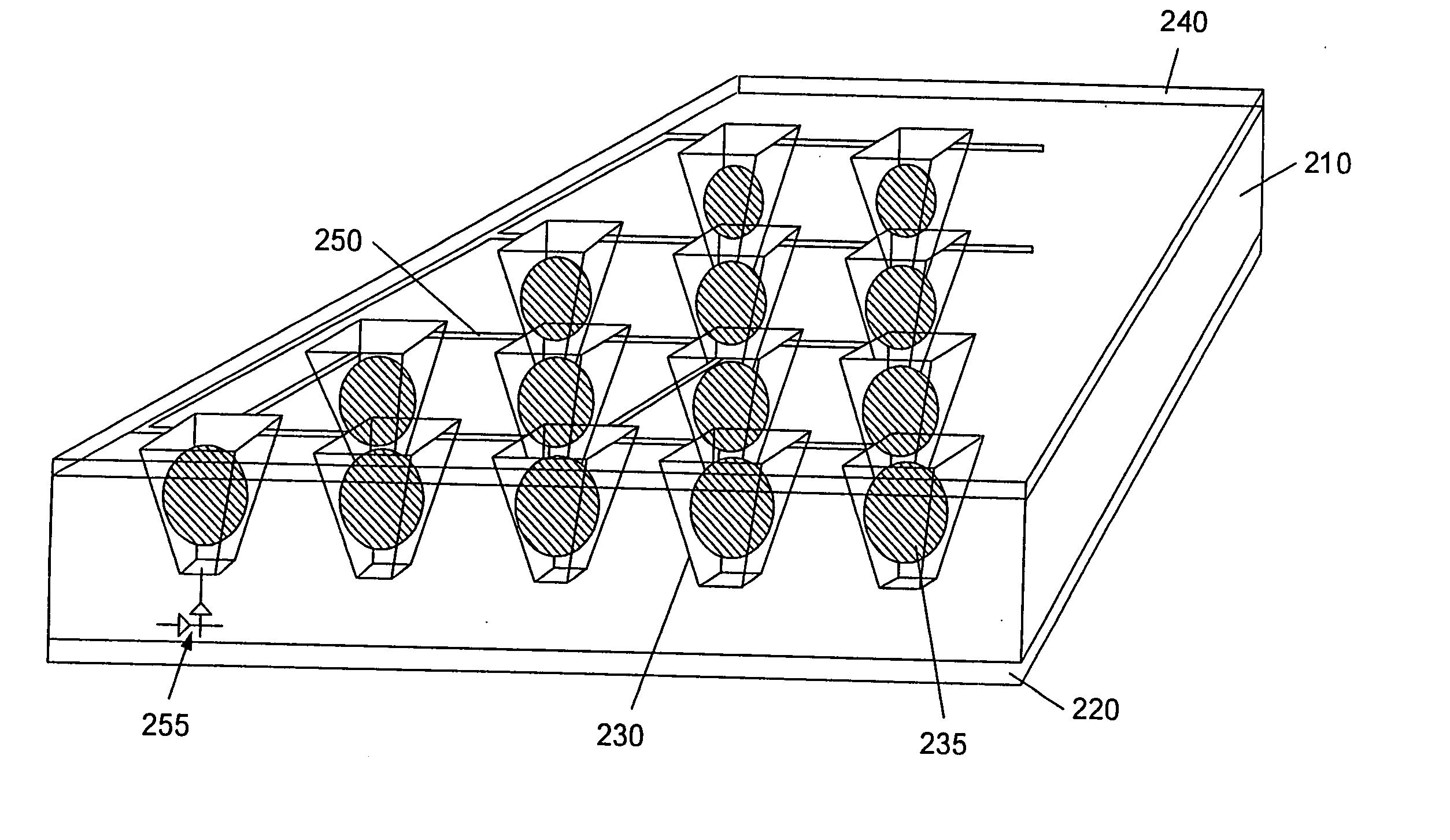
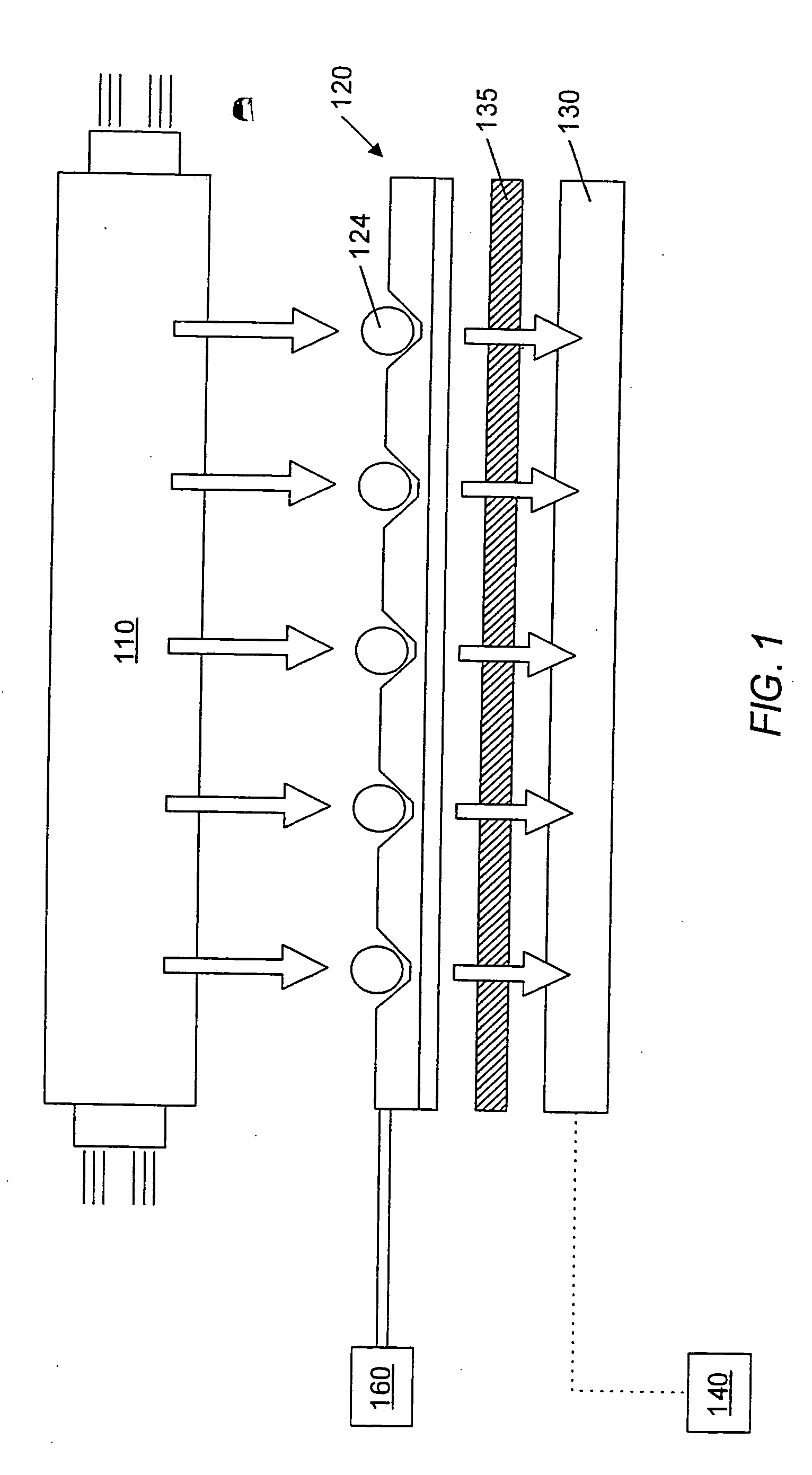
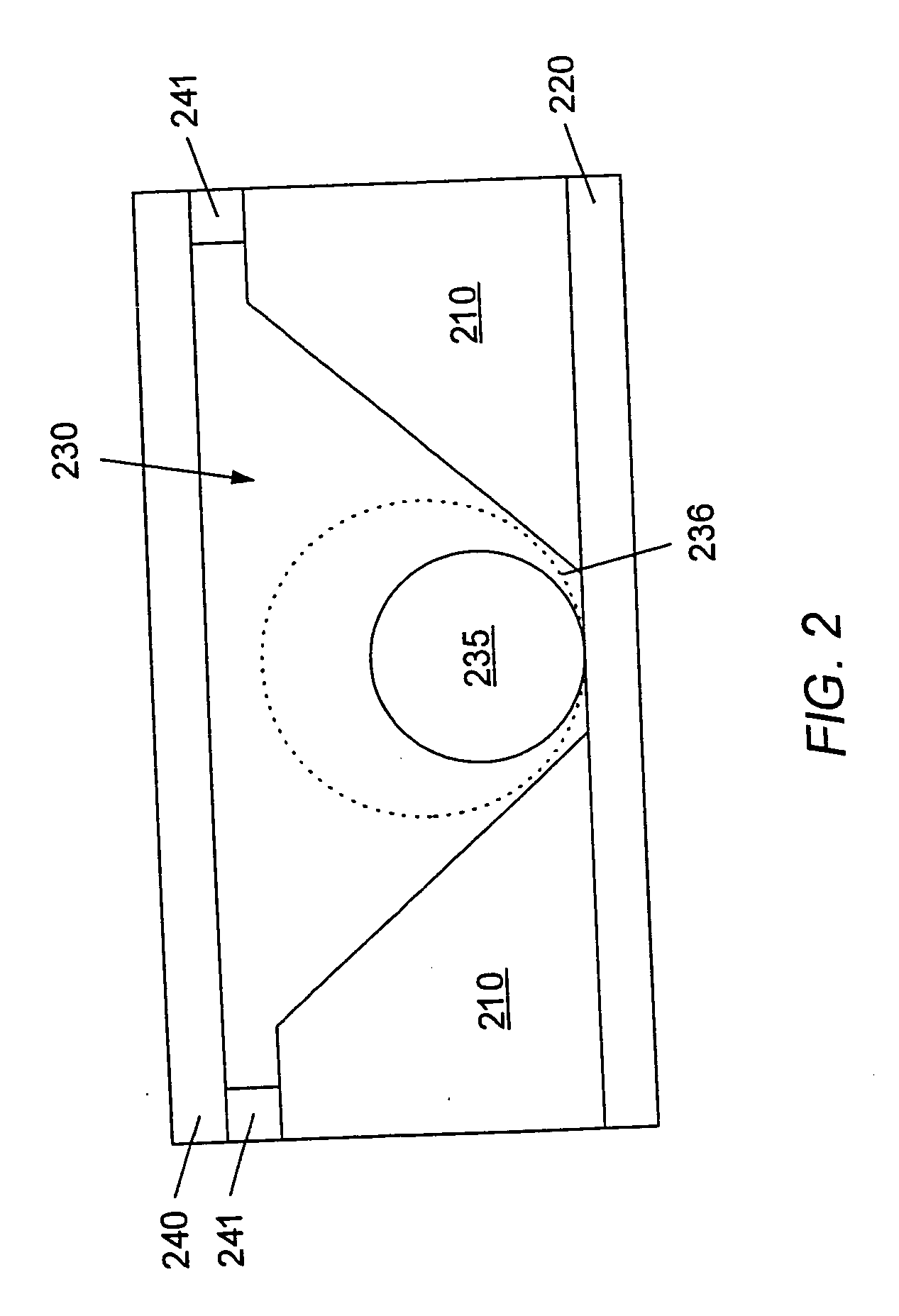
System and method for the analysis of bodily fluids
a bodily fluid and system technology, applied in the direction of fluid speed measurement, special data processing applications, optical light guides, etc., can solve the problems of small changes in the electrical resistance of these layers, preventing the creation of large saw arrays with multiple sensor sites, and reducing the detection efficiency of fluids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0495] In the below recited table are examples of analytes that have been detected using the sensor array system described herein. In the Receptor / Enzyme column are listed examples of receptors that may be used for the corresponding analyte. These receptors are covalently bound to a polymeric resin, using methods described herein.
AnalyteTypeReceptor / EnzymeSodium, PotassiumSmall Molecule (Electrolyte)Crown ethers, cryptands,chromoionophores such as Chromolyte ®(from Bayer), Enzymes such as β-galactosidase, or other metalloenzymes.BicarbonateSmall Molecule (Electrolyte)Enzymes such as Carbonic anhydraseCalciumSmall Molecule (Electrolyte)Complexometric dyes such as ArsenazoIII, Xylenol Orange, AlizarenComplexoneMagnesiumSmall Molecule (Electrolyte)Complexometric dyes such as Calmagite,MagonChlorideSmall Molecule (Electrolyte)Enzymes and / or small molecule detectorssuch as Amylase, Phenyl mercurycompounds, mercuric thiocynanates,diphenylcarbazonesOxygenSmall Molecule (Metabolite)Oxygen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com