Gene delivery vectors provided with a tissue tropism for smooth muscle cells, and/or endothelial cells
a tissue tropism and gene technology, applied in the field of biotechnology, can solve the problems of insufficient in vivo delivery capacity of adenovirus vectors, limited use of current vectors in specific applications, and inability to easily transduce endothelial cells and smooth muscle cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Generation of Adenovirus Serotype 5 Based Viruses with Chimeric Fiber Proteins
Generation of Adenovirus Template Clones Lacking DNA Encoding for Fiber
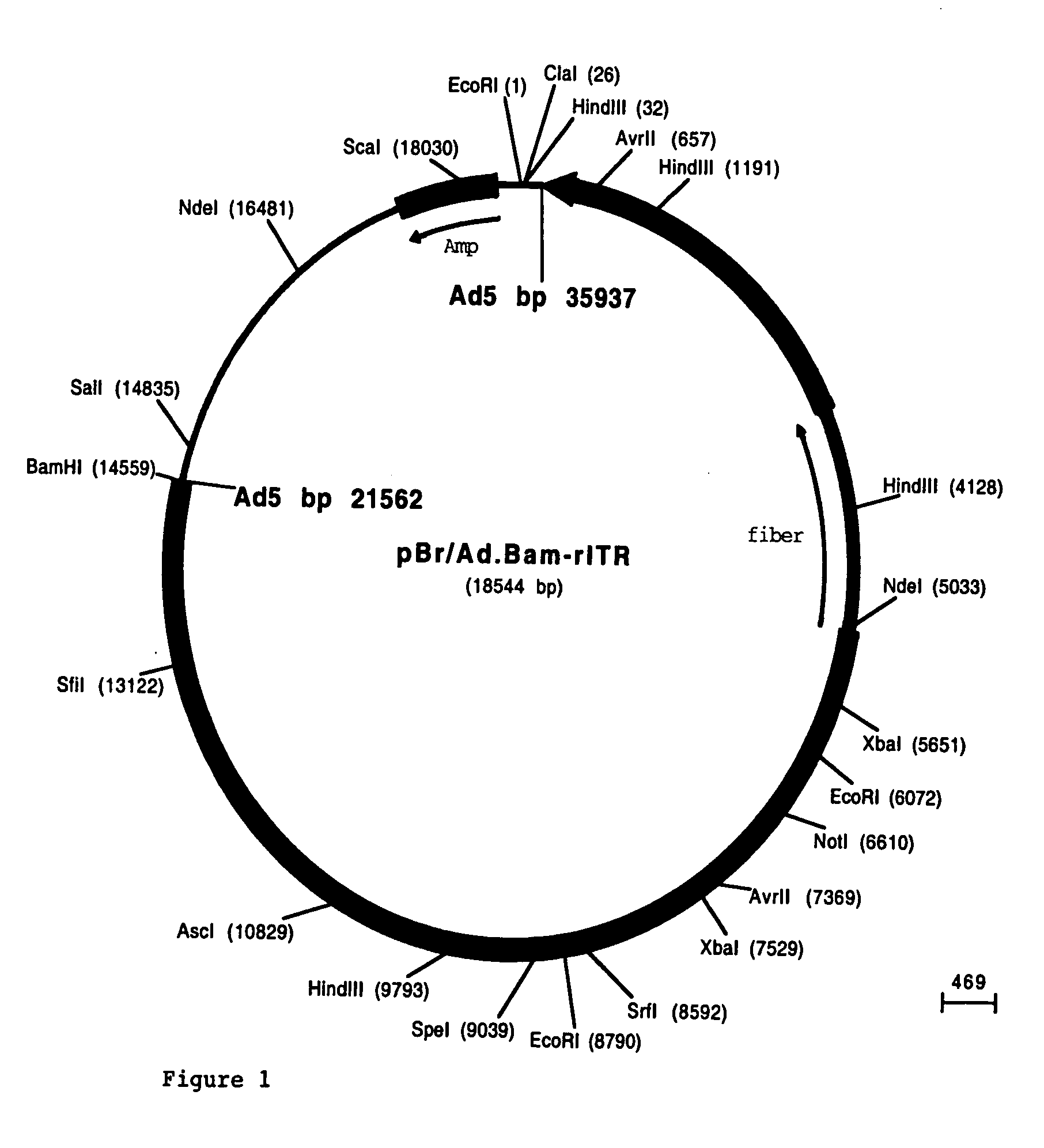
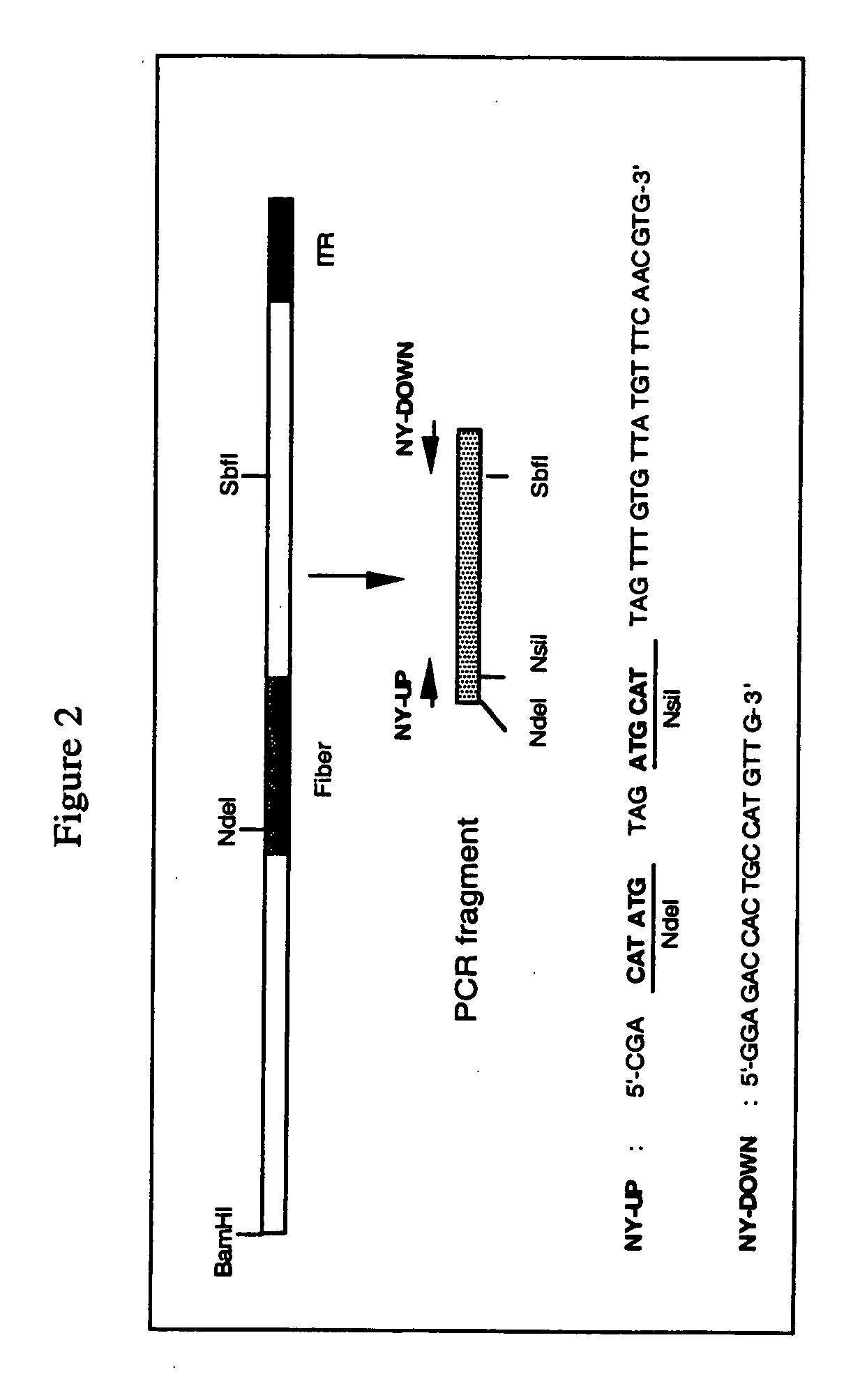
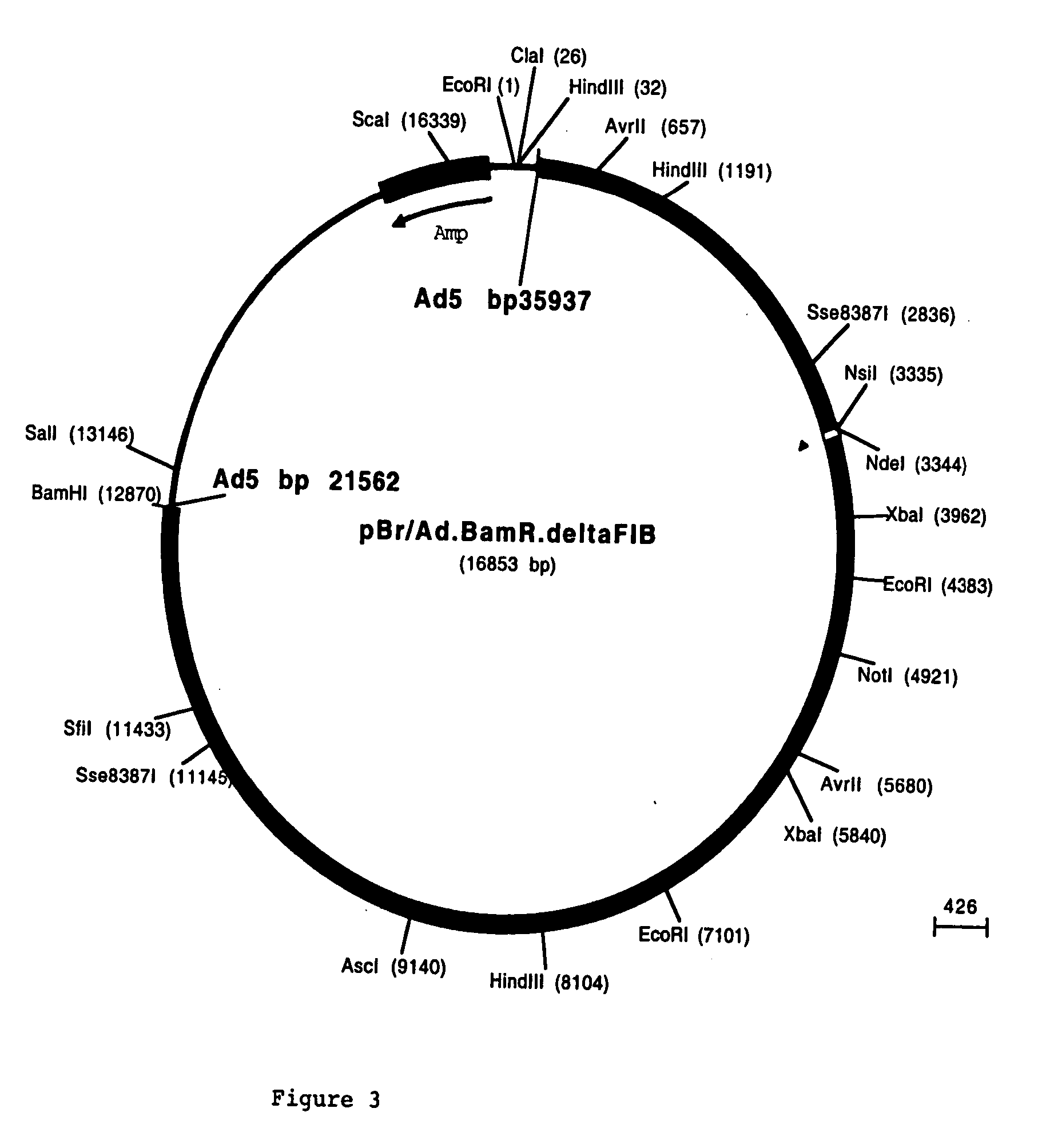
[0094] The fiber coding sequence of adenovirus serotype 5 is located between nucleotides 31042 and 32787. To remove the adenovirus serotype 5 DNA encoding fiber we started with construct pBr / Ad.Bam-rITR (FIG. 1; ECACC deposit P97082122). From this construct a NdeI site was first removed. For this purpose, pBr322 plasmid DNA was digested with NdeI after which protruding ends were filled using Klenow enzyme. This pBr322 plasmid was then re-ligated, digested with NdeI and transformed into E. coli DH5α. The obtained pBr / ΔNdeI plasmid was digested with ScaI and SalI and the resulting 3198 bp vector fragment was ligated to the 15349 bp ScaI-SalI fragment derived from pBr / Ad.Bam-rITR, resulting in plasmid pBr / Ad.Bam-rITRΔNdeI which hence contained a unique NdeI site. Next a PCR was performed with oligonucleotides “NY-up” and “NY-down” (FIG....
example 2
Biodistribution of Chimeric Viruses after Intravenous Tail Vein Injection of Rats
[0099] To investigate the biodistribution of the chimeric adenoviruses carrying fiber 12, 16, 28, or 40-2, 1×1010 particles of each of the generated virus batches was diluted in 1 ml PBS after which the virus was injected in the tail vein of adult male Wag / Rij rats (3 rats / virus). As a control, Ad5 carrying the luciferase transgene was used. Forty-eight hours after the administration of the virus, the rats were sacrificed after which the liver, spleen, lung, kidney, heart, and brain were dissected. These organs were subsequently mixed with 1 ml of lysis buffer (1% Triton X-100 / PBS) and minced for 30 seconds to obtain a protein lysate. The protein lysate was subsequently tested for the presence of transgene expression (luciferase activity) and the protein concentration was determined to express the luciferase activity per μg of protein. The results, Shown in Table II, demonstrate that in contrast to the...
example 3
Chimeric Viruses Display Differences in Endothelial and Smooth Muscle Cell Transduction
A) Infection of Human Endothelial Cells
[0100] Human endothelial cells (HUVEC) were isolated, cultured and characterized as described previously (Jaffe et al. 1973; Wijnberg et al. 1997). Briefly, cells were cultured on gelatin-coated dishes in M199 supplemented with 20 mM HEPES, pH 7.3 (Flow Lab., Irvine, Scotland), 10% (v / v) human serum (local blood bank), 10% (v / v) heat-inactivated newborn calf serum (NBCS) (GIBCO BRL, Gaithersburg, Md.), 150 μg / ml crude endothelial cell growth factor, 5 U / ml heparin (Leo Pharmaceutics Products, Weesp, The Netherlands), penicillin (100 IU / ml) / streptomycin (100 μg / ml) Boehringer Mannheim, Mannheim, Del.) at 37° C. under 5% (v / v) CO2 / 95% (v / v) air atmosphere. Cells used for experiments were between passage 1-3. In a first set of experiments 40000 HUVEC cells (a pool from 4 different individuals) were seeded in each well of 24-well plates in a total volume of 20...
PUM
| Property | Measurement | Unit |
|---|---|---|
| total volume | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com