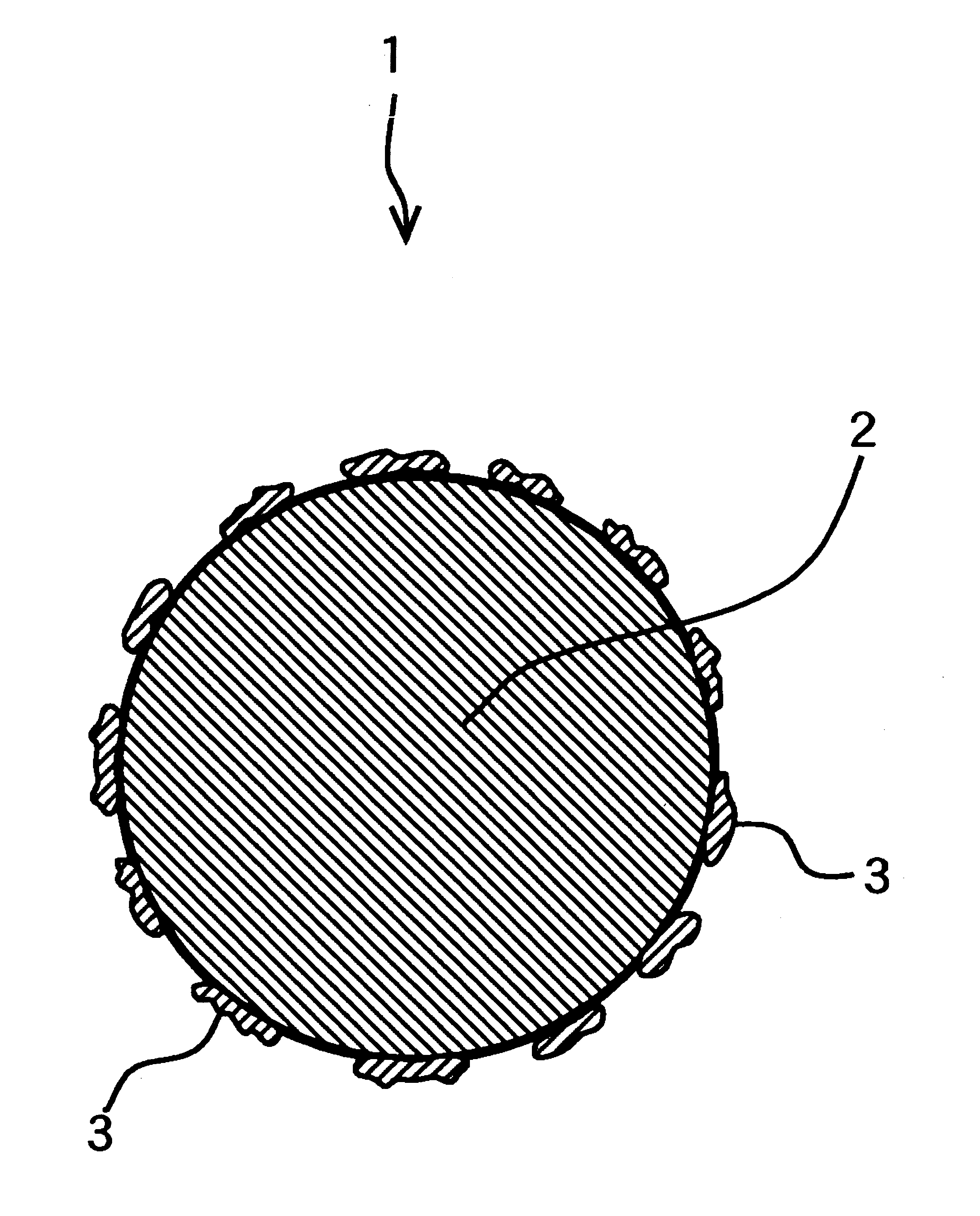
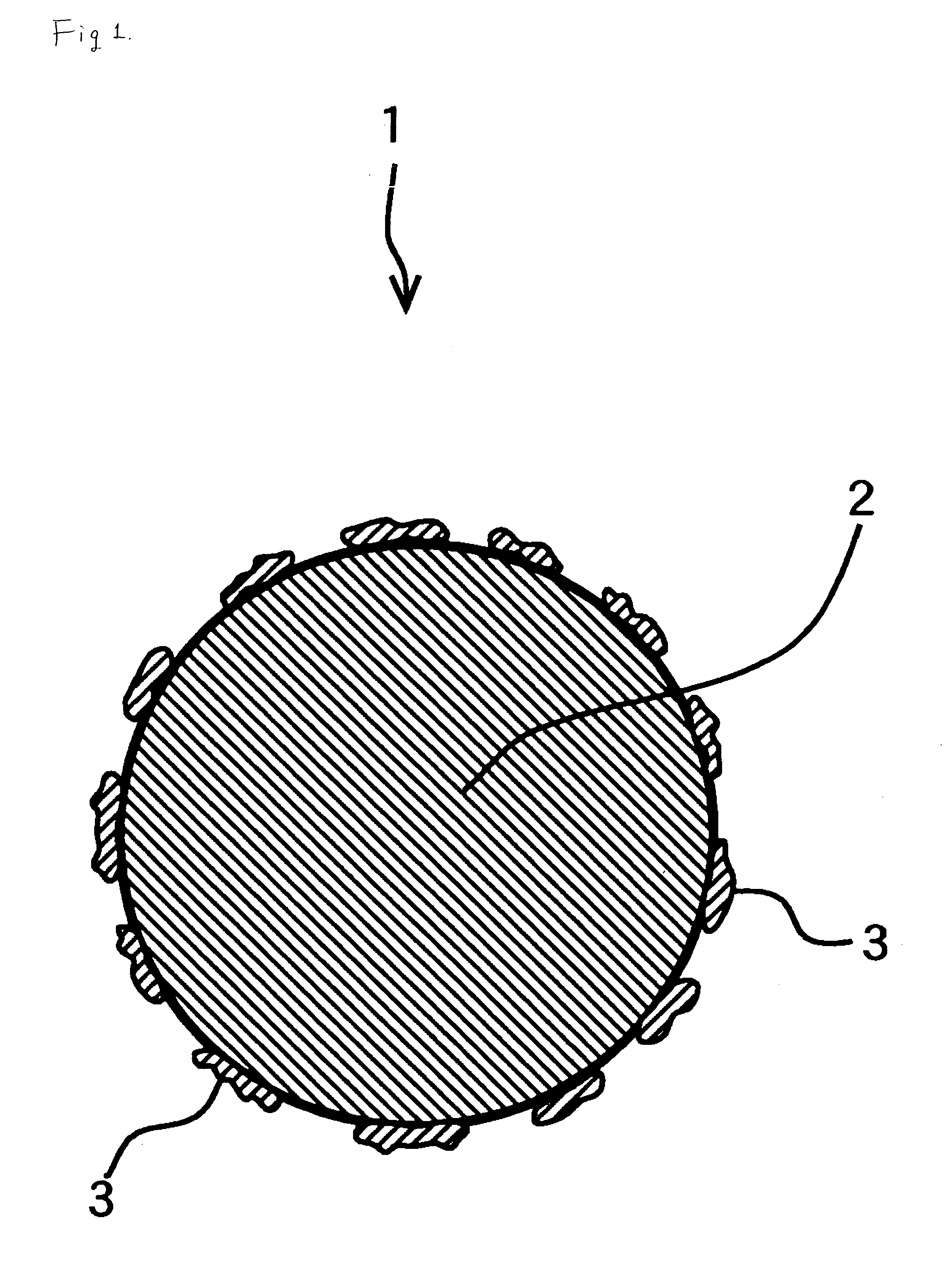
Silver powder made of silver particles, each to which fine silver particles adhere and process of producing the same
a silver nanoparticle and silver powder technology, applied in the direction of printed circuit aspects, semiconductor/solid-state device details, transportation and packaging, etc., can solve the problem of difficult to meet the size-refinement of powder particles and the dispersibility meant by powder particles nearly in a mono-disperse state, and the low-temperature sintering performance of silver nanoparticles cannot be used to the full, so as to reduce the amount of impurities contained, improve the effect of low-temperature sintering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Process for Producing Silver Powder Used as a Core Material:
[0068] In this example, first silver powder (whose particle is spherical) used as a core material was produced. The production process was as follows.
[0069] First, 63.3 g of silver nitrate was dissolved in 9.7 liter of deionized water to prepare an aqueous solution of silver nitrate. Then, 235 ml of 25% by weight aqueous ammonia was added for a very short time to the aqueous solution of silver nitrate and stirred to give an aqueous solution of a silver ammine complex.
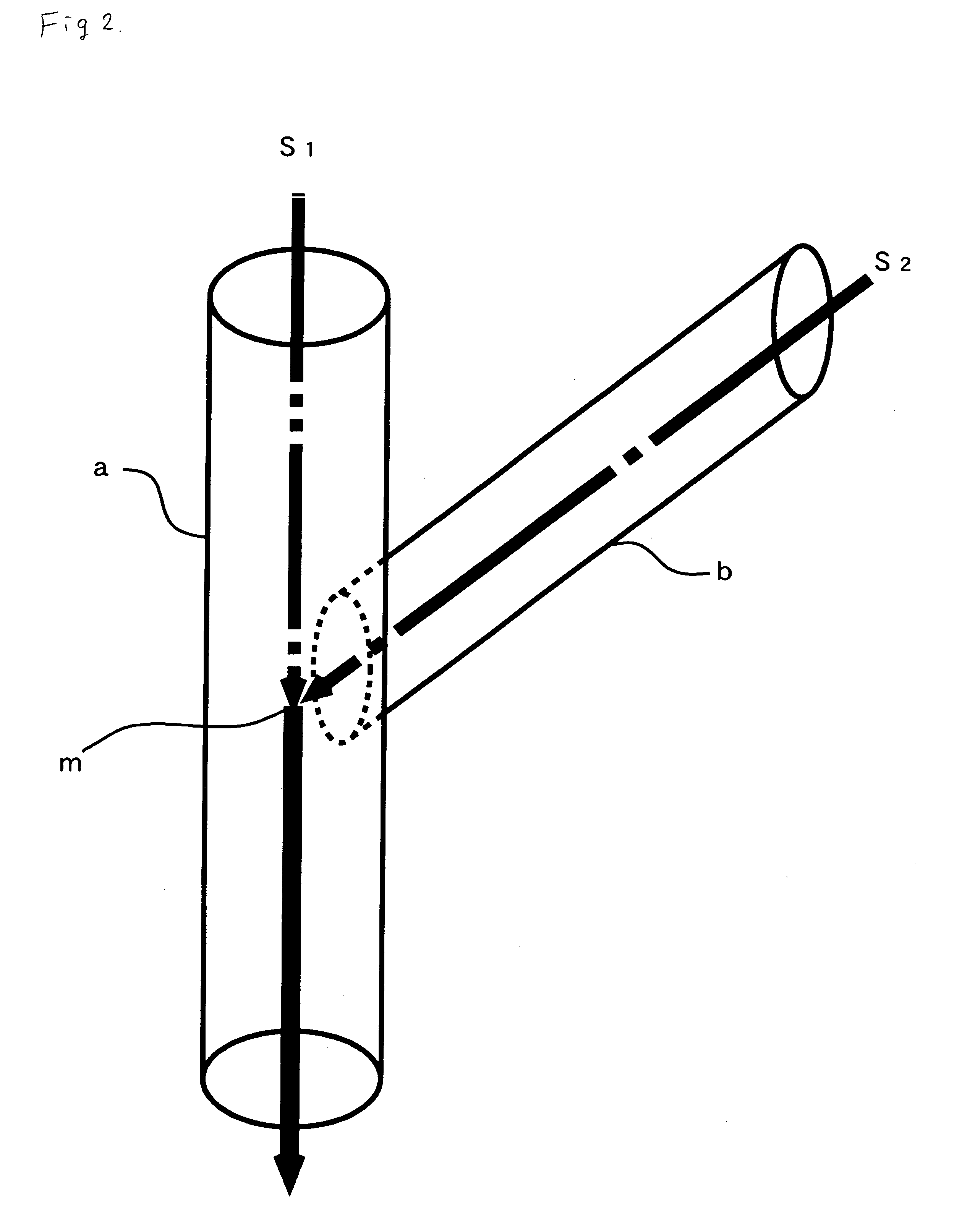
[0070] The aqueous solution of a silver ammine complex was introduced into a first pass a having an inner diameter 13 mm, shown in FIG. 2, at a flow rate of 1500 ml / sec and a reducing agent was allowed to flow through a second pass b at a flow rate of 1500 ml / sec so that the solution and the agent came into contact with each other at a juncture m while being kept at 20° C. to precipitate fine silver powder through reduction. The reduction agent used here wa...
example 2
Process for Producing Silver Powder Used as a Core Material:
[0077] In this example, first silver powder (which is substantially spherical) used as a core material was produced. The production conditions were as described below.
[0078] Silver powder was produced under production conditions different from those of Example 1 and the powder characteristics of the resultant silver powder were determined. Then, a silver paste was prepared using the above silver powder and a test circuit was formed using the silver paste. Then, the specific resistance of the conductor and sinterable temperature were measured for the formed test circuit.
[0079] First, 63.3 g of silver nitrate was dissolved in 3.1 liter of deionized water to prepare an aqueous solution of silver nitrate. Then, 235 ml of 25% by weight aqueous ammonia was added for a very short time to the aqueous solution of silver nitrate and stirred to give an aqueous solution of a silver ammine complex.
[0080] The aqueous solution of a s...
example 3
Process for Producing Silver Powder Used as a Core Material:
[0087] In this example, first silver powder (of nearly spherical shape) having a large crystallite size was produced using the process shown below, and the powder characteristics of the resultant silver powder were determined. Then, a silver paste was prepared using the above silver powder and a test circuit was formed using the silver paste. Then the specific resistance and sinterable temperature were measured for the formed test circuit.
[0088] First, 20 g of polyvinyl pyrrolidone was dissolved in 260 ml of deionized water and 50 g of silver nitrate was dissolved to prepare an aqueous solution of silver nitrate. Then, 25 g of nitric acid was added for a very short time to the above solution and stirred to yield a silver-containing nitric acid solution. At the time of completion of the mixing, the concentration of ascorbic acid was about 36.0 g / l.
[0089] A reducing solution was prepared by adding and dissolving 35.8 g of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| crystallite size | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com