Methods for accelerating bone, cartilage, and connective tissue growth
a technology of connective tissue and bone, which is applied in the direction of skeletal/connective tissue cells, parathyroid hormones, osteogenic factors, etc., can solve the problems of individual at risk of skeletal failure, slow process, and requiring years to reunite, and achieve the effect of enhancing bone and cartilage repair
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Method for Culture of Rabbit Chondrocytes
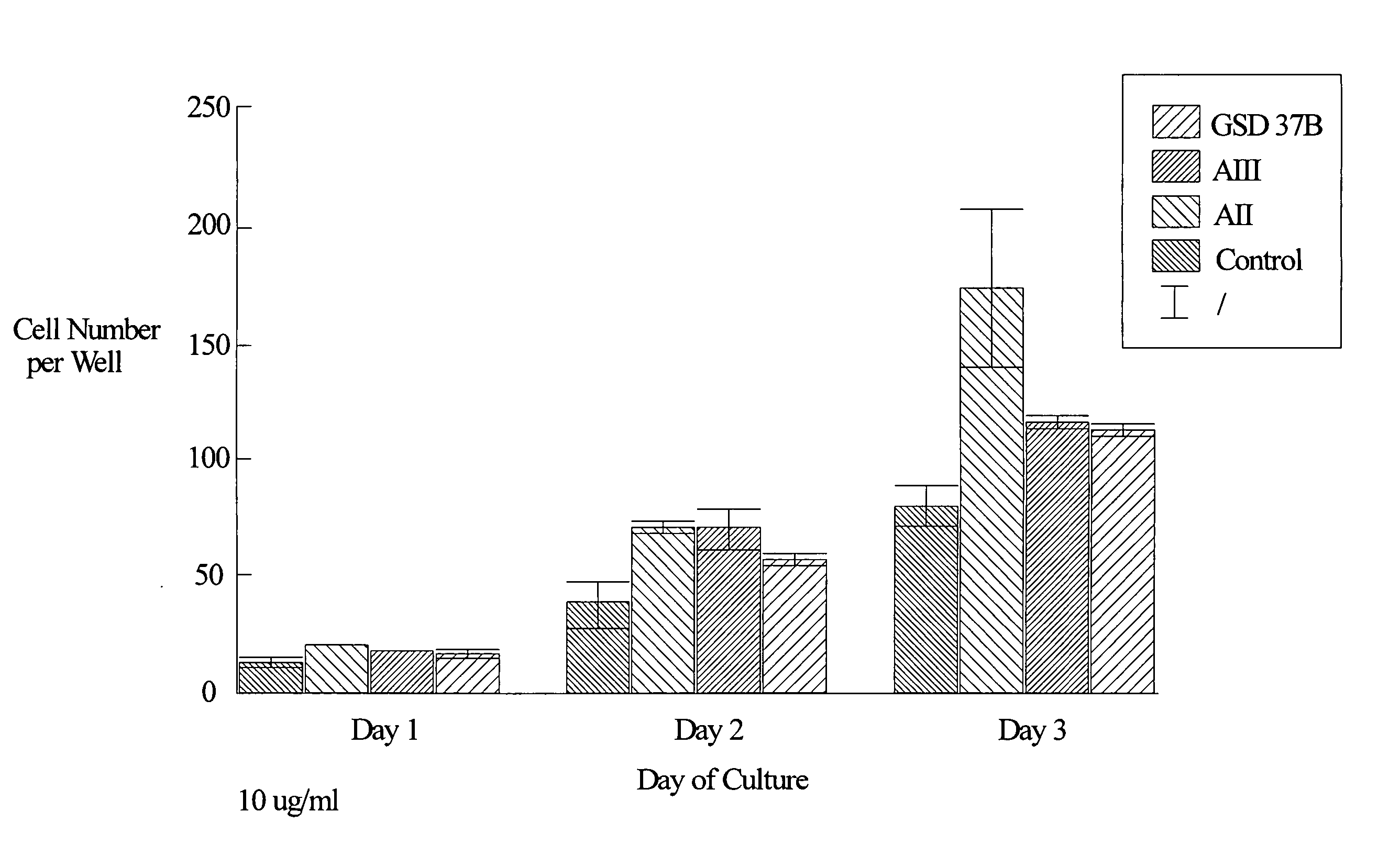
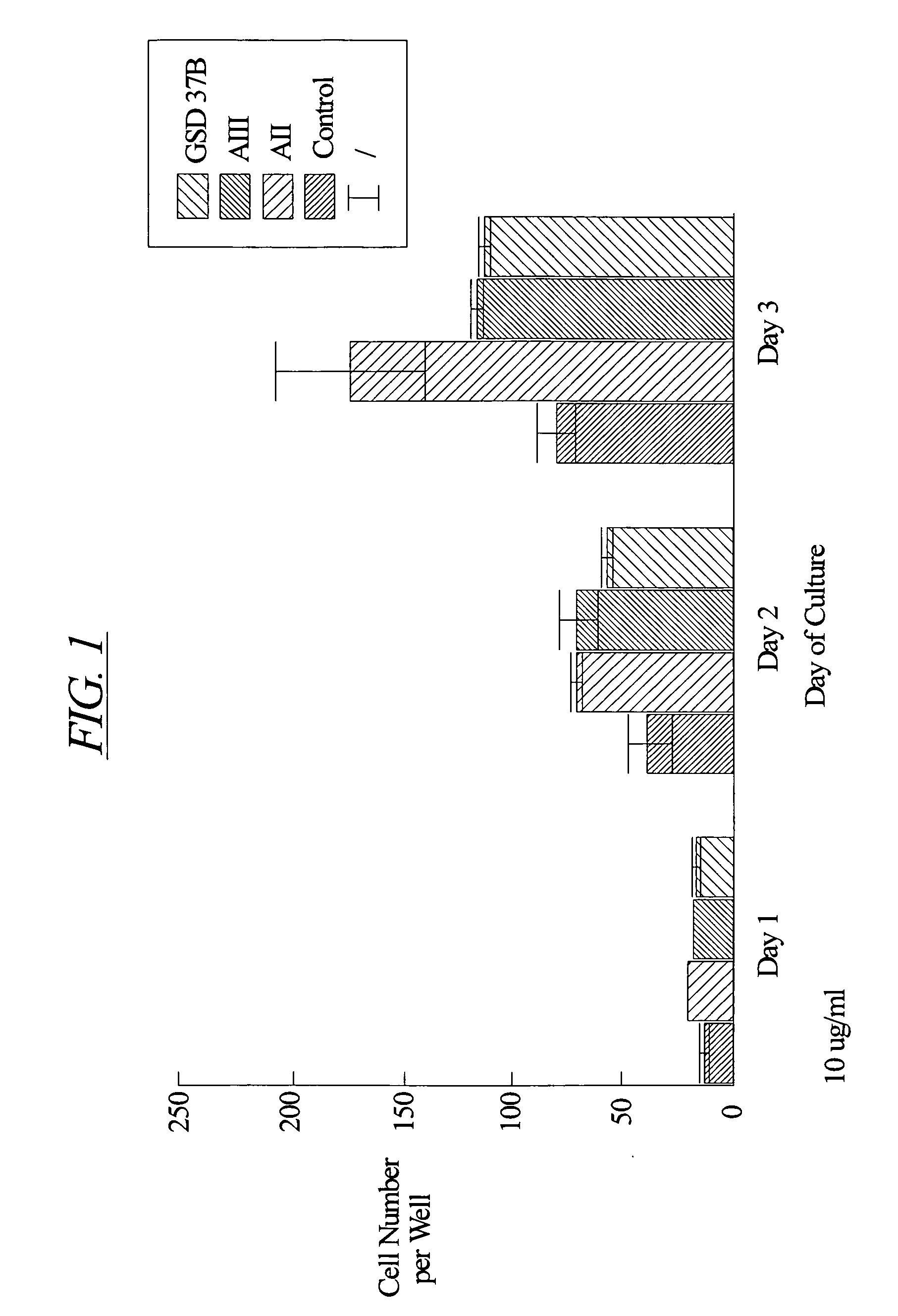
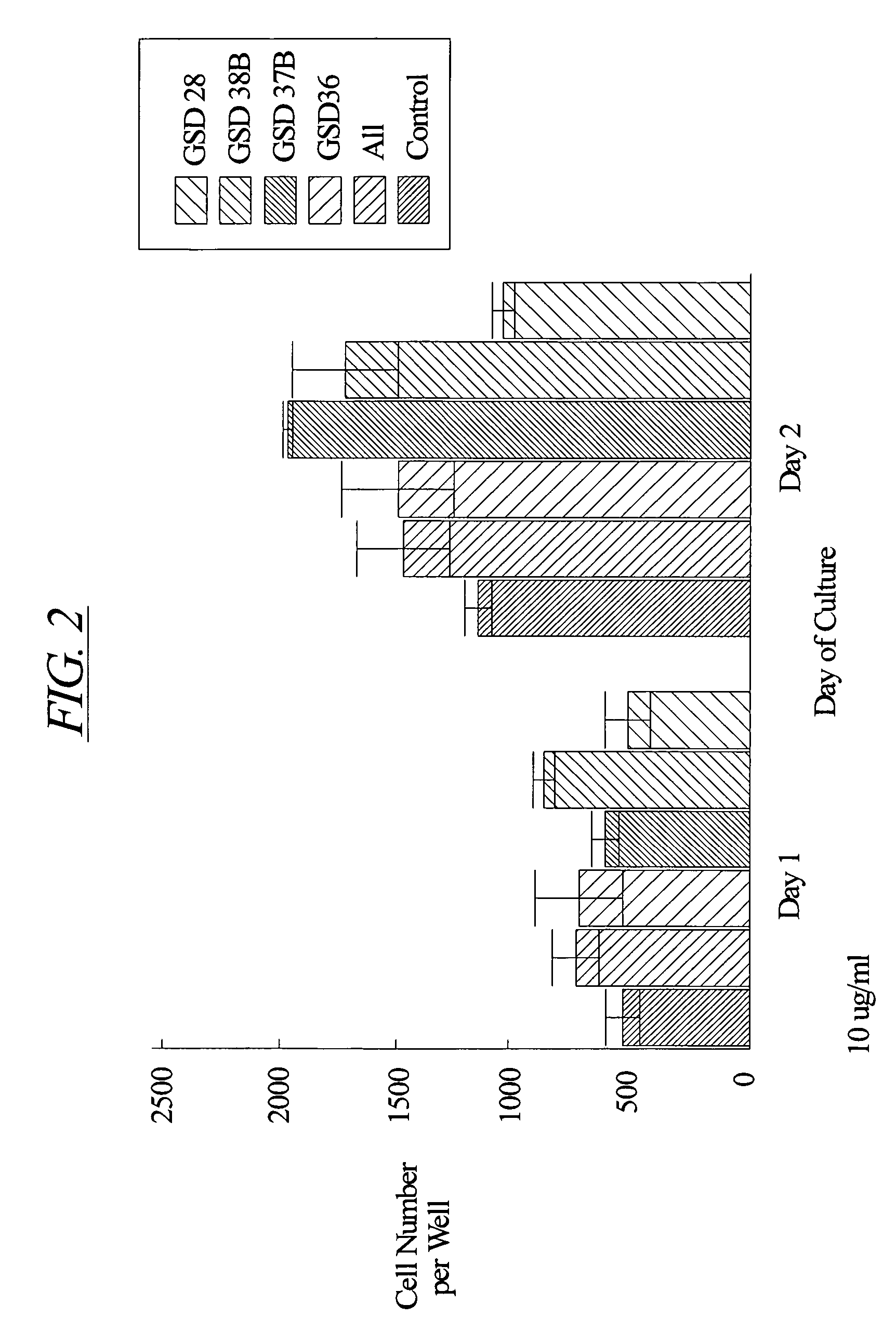
[0126] Chondrocytes were isolated from the cartilage of the knee joints of adult rabbits and cultured according to the method of Okazaki et al. (Ann. Rheum. Dis. 55:181-186, 1996). Briefly, cartilage explants were minced into small pieces (approximately 1.5 mm by 1.5 mm). The tissue pieces were digested at 37° C. sequentially in 0.05% hyaluronidase (415 U / mg protein) for 10 minutes, 0.2% Type III trypsin (10,000 U / mg protein, Sigma) and 0.53 mM EDTA for 15 minutes, and 0.2% Type I collagenase (136 U / mg protein, Sigma) for 30 minutes. The samples were then washed and incubated overnight at 37° C. in 5% CO2 in air in Dulbecco's modified Eagle's medium enriched with 10% fetal calf serum, 3.5 mg / ml glucose, 0.2% Type I collagenase and antibiotics (100 U / ml penicillin and 100 μg / ml streptomycin).
[0127] After this incubation, the cells were harvested and washed once with phosphate buffered saline (pH 7.2). The cells were then counted with a hemac...
example 2
[0128] Female, Sprague Dawley rats underwent intramuscular anesthesia with ketamine / rompum and were prepared for sterile surgery by shaving the surgical site and scrubbing with Betadine scrub followed by 70% ethanol. The rat was then placed on a sterile field in a lateral decubitis position facing the surgeon. The shaved legs were then covered with Betadine solution and draped aseptically. A skin incision was performed parallel to the long axis of the right medial diaphysis. The muscle was separated along fascial planes to expose the tibia. A defect of 1.4 mm in diameter was then drilled from the lateral side of the midshaft cortex so that the defect extended from one cortical side to the other, through the bone marrow. Sterile saline (0.9% NaCl) for injection was then used to clean the surgical area of tissue debris and bone fragments. Either hydron polymer solution (vehicle: 10% Hydron, 60% ethanol, 1% polyethylene glycol polymer) or peptide (AII, AII(1-7), or 9GD at ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com