Immune responses using compositions containing stress proteins
a technology of immune responses and compositions, applied in the direction of antibody medical ingredients, peptide/protein ingredients, peptide sources, etc., can solve the problems of high frequency of viral genome mutations, lack of proof-reading capability of transcriptionase activities responsible for these steps, and high recovery rate, etc., to suppress allergic immune responses, and suppress allergic immune responses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Isolation of Recombinant Stress Proteins
[0086] A. Recombinant Mycobacterial Hsp70. Plasmid Y3111 contains an M. tuberculosis Hsp70 gene functionally inserted between expression control sequences. (Mehlert, A. and Young, D. B., Mol. Microbiol., 3:125-130 (1989). E. coli strain CG2027 (obtained from C. Georgopoulos, University of Geneva, Switzerland) containing a truncated Hsp70 gene was transformed with plasmid Y3111 by standard procedures. (Maniatis, et al., Molecular Cloning, A Laboratory Manual, Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y. (1982)).
[0087] Bacteria containing plasmid Y3111 were grown overnight in 2×YT medium (20 g Tryptone, 10 g yeast extract, 10 g NaCl per liter) containing 100 microgram / ml ampicillin at 37° C., with agitation (250 rpm). A 10% glycerol stock was prepared from this culture and was stored at −70° C. Several scrapings from the frozen glycerol stock were used to inoculate a large culture that was incubated as before for about 48 h. When th...
example 2
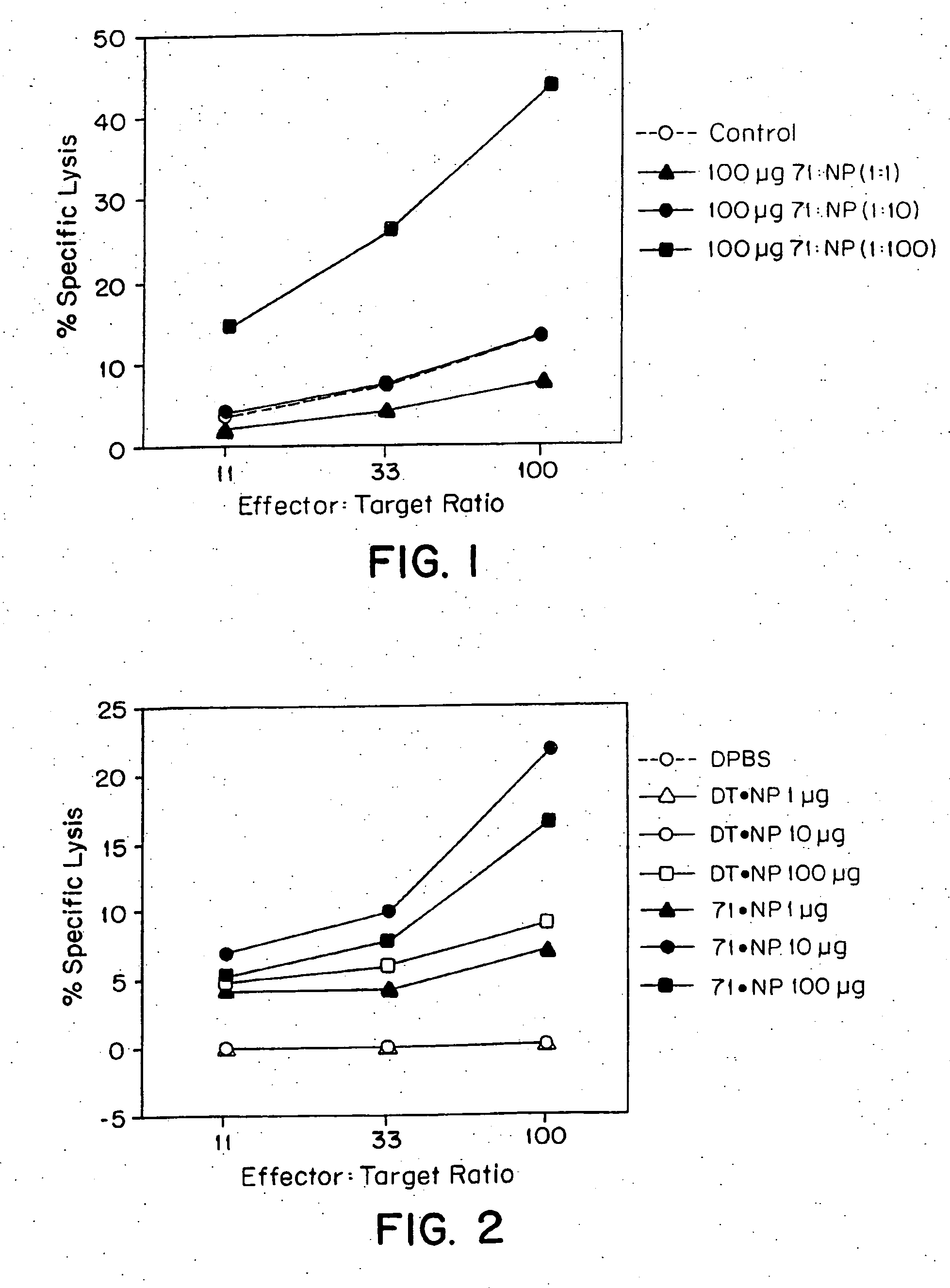
CTL response to a Composition Comprising a Mixture of an NP Peptide and hsp70
[0101] a. Preparation of hsp70 and NP peptide Hsp 70, here M. tuberculosis hsp71, was prepared as described in example 1. NP peptide (referred to herein as NP.B; Motal, U. M. A., et al., Eur. J. Immunol., 25:1121-1124 (1995) and references therein) with the amino acid sequence VQLASNENMETM (SEQ ID NO: 1) corresponding to residues 363-374 in the complete NP and containing a known CTL epitope (H-2b-restricted) was produced synthetically (0.25 mM scale) on an Applied Biosystems model 431A peptide synthesizer using Fmoc (9-fluorenylmethyloxycarbonyl) as the alpha-amino protective group and HMP (Wang) resin as the solid support. All amino acid and synthesis chemicals were purchased from Applied biosystems.
[0102] NP.B was cleaved off the support and side-chain-protecting groups were removed by incubating under continuous agitation NP.B-resin for 3 h in 2 ml of a mixture prepared by combining 10 ml trifluoroacet...
example 3
CTL Response to a Composition Comprising a Chemical Conjugate of an NP Peptide and hsp70
a. Preparation of hsp70 and NP Peptide
[0106]M. tuberculosis hsp71 was prepared as described in Example 1. NP.B peptide was synthesized as discussed in Example 2, except that the peptide contained an extra amino-terminal cysteine residue and, thus, had the amino acid sequence CVQIASNENMETM (SEQ ID NO: 2).
b. Chemical Conjugation of Np.B Peptide to hsp70 and Diphtheria Toxoid
[0107] Conjugations were carried out with both hsp70 and, to provide a standard for comparisons of efficacies of specific stimulation of CTL activity, commonly used carrier protein diphtheria toxoid (abbreviated DT; DT was obtained from a commercial source).
b.1. Activation of M. tuberculosis hsp71 and DT Carrier Proteins
[0108] Nine mg of hsp71 were dissolved in 4.5 ml of 0.1 M sodium borate buffer, pH 8.0. Sulfo-MBS (m-maleimidobenzoyl-N-hydroxysulfosuccinimide ester) (2.3 mg in 100 ul dimethyl sulfoxamine) was added to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Immunogenicity | aaaaa | aaaaa |
| Stress optical coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com