Probiotic strain for the production of the hyaluronic acid and use thereof
A hyaluronic acid and strain technology, applied in the field of novel probiotic strains, can solve problems such as zoonotic disease virus infection, and achieve the effects of increasing the number, promoting lymphocyte activity, and anti-inflammatory lymphocyte activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1 isolates bacterial strain from breast milk
[0030] Streptococcus thermophilus TCI633 (deposit number DSM28121) was isolated from healthy human breast milk. Firstly, fresh breast milk samples were diluted and smeared on MRS-Cys (cysteine) agar plates to isolate Lactobacillus spp. and Streptococcus spp. Under the conditions for 48 hours, identify the colony (colony) type with a pictorial book, select at least 1 representative strain from each colony type on the agar plate, and select 5 to 10 strains with different colony types in total, and use a microscope and Gram Staining to predict possible strains.
Embodiment 2
[0031] Example 2 screening strains
[0032] 2.1 Methods of biochemical identification:
[0033] Gram's stain, the washed cells were fixed with 10% neutral formalin for 30 minutes, washed 4 times with phosphate buffer, and the cells in culture and the strains to be tested were subjected to Gram's staining. dyeing.
[0034] 2.2 Molecular identification methods:
[0035] Because different species of microorganisms must have their unique nucleic acid sequences, as far as bacteria are concerned, the 16S rDNA gene sequence differences of the same strain are very small, so the present invention is used to identify strains.
[0036] The present invention utilizes 16S DNA Broad-Range polymerase chain reaction (Simo Nikkari, Fred A. Lopez et al., Emerging Infectious Disease Journal 2002, 8, 188-194) detection to confirm the isolated strains. Use taco first TM Total DNA Extraction Kit (Ruiji Marine Biotechnology Co., Ltd., Taiwan) extracted the total DNA of the isolated strains. The 16...
Embodiment 3
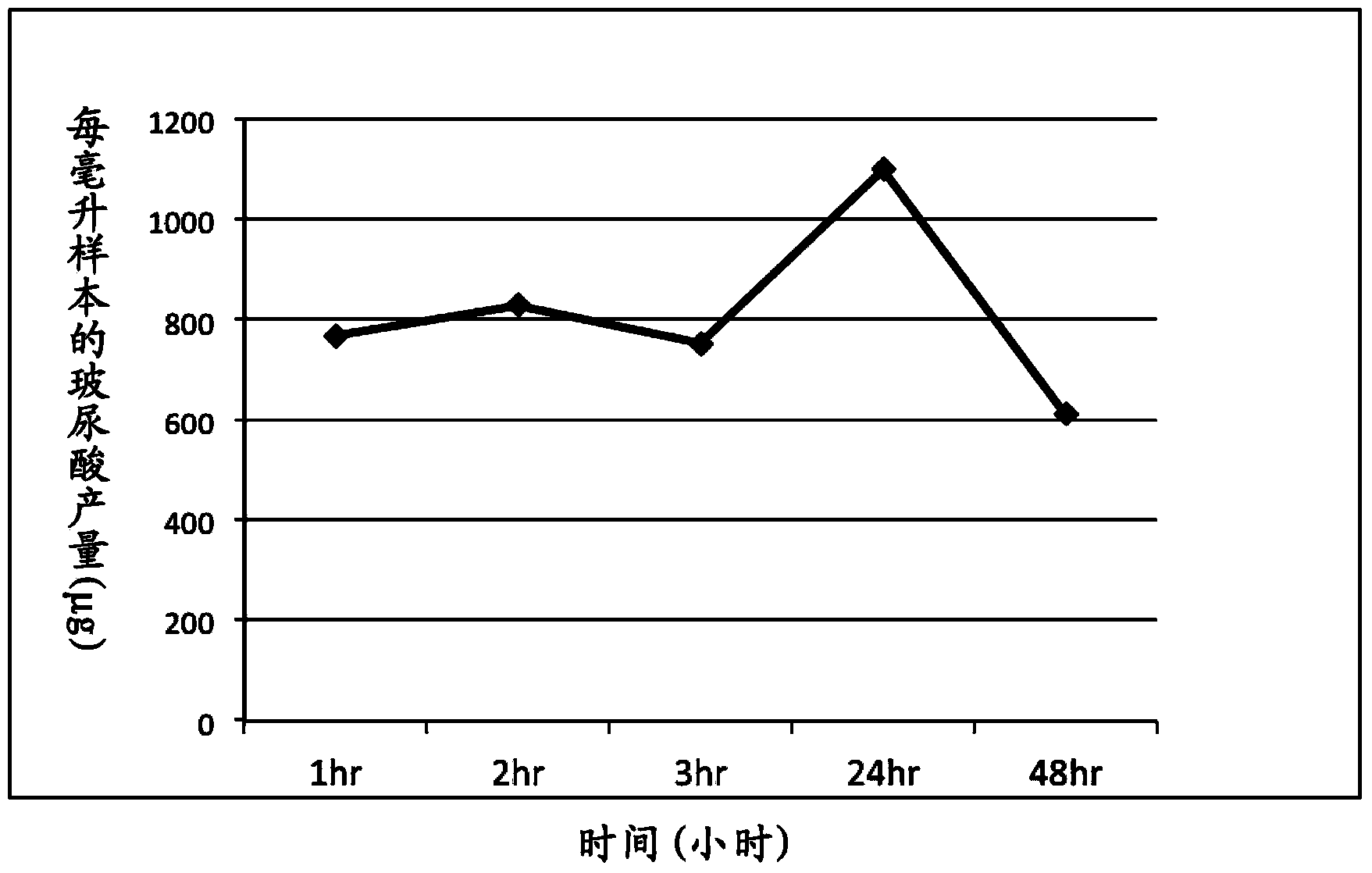
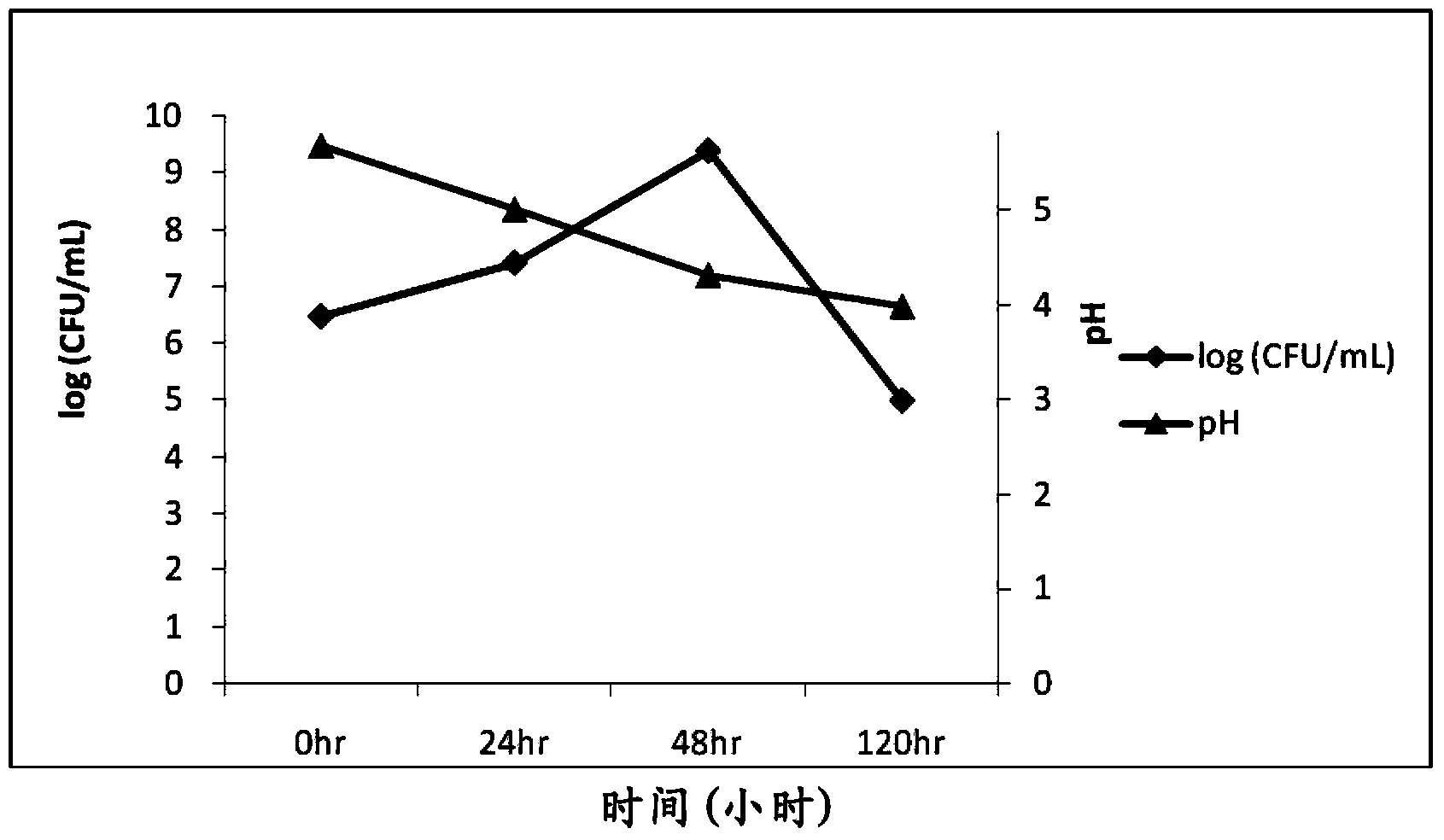
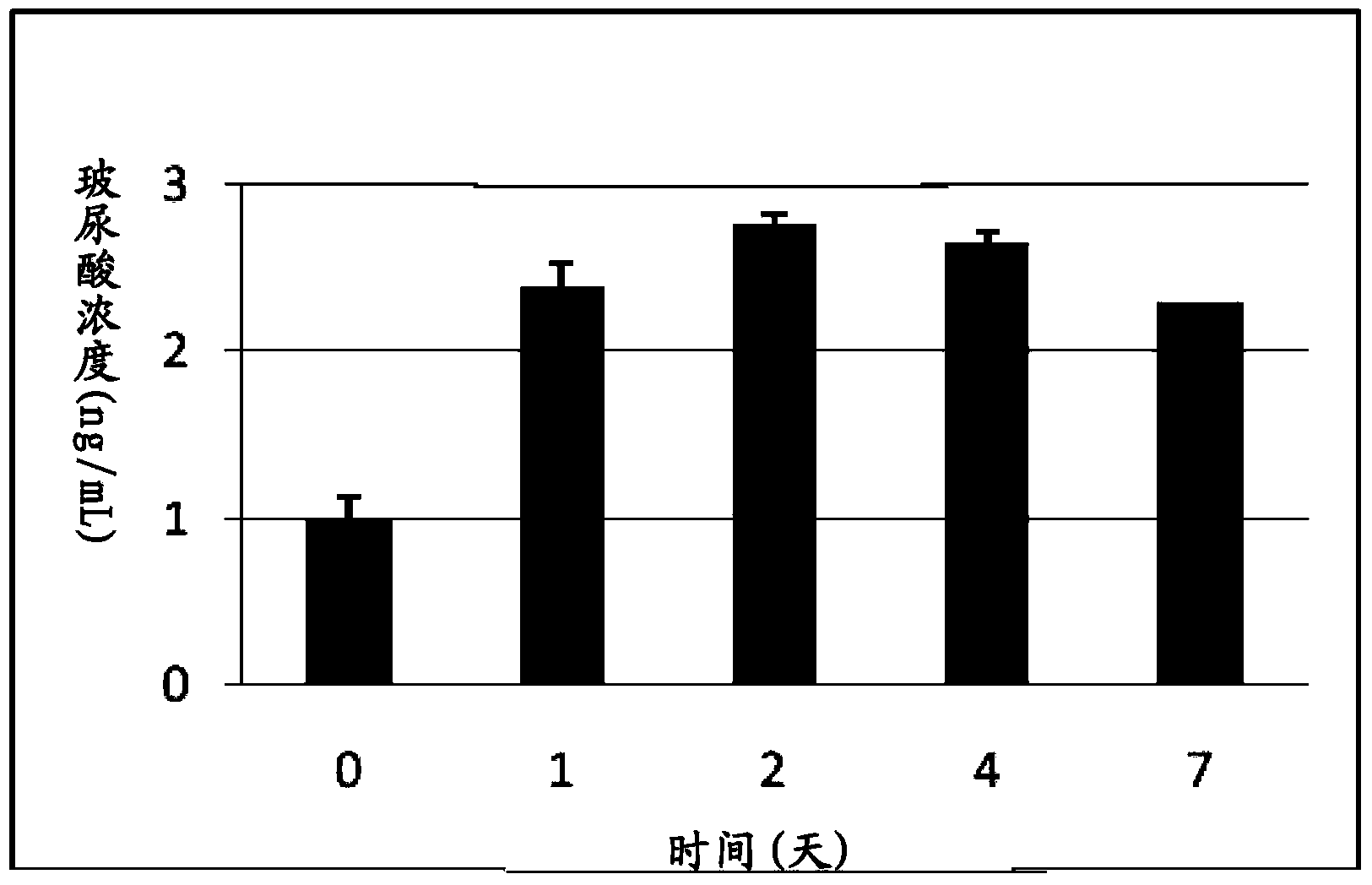
[0038] Example 3 Artificial Intestinal Fluid (AIS) Simulation Experiment (in vitro)
[0039] The artificial intestinal juice was modified according to the formula proposed by Hasjim et al. (Jovin Hasjim, Gautier Cesbron Lacau et al., Biomacromolecules 2010, 11, 3600-3608). Digestive enzymes including pancreatin (8mg / mL), α-amylase (13U / mL) and amyloglucosidase (1.12U / mL) were added to 0.2M acetate buffer (0.49mM MgCl 2 And 200mM calcium chloride, pH6.0), as artificial intestinal juice. The sterilized MRS medium is then added with artificial intestinal fluid to simulate the nutrients that bacteria grow in the intestinal tract and will ingest daily food. Then, TCI633 is added for culture and cultured at a human body temperature of 37°C to establish an artificial intestinal fluid environment. The bacterial fluid was collected at different time points to monitor the growth of the probiotic strains and the production of hyaluronic acid.
[0040] The plate counting method was used...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com