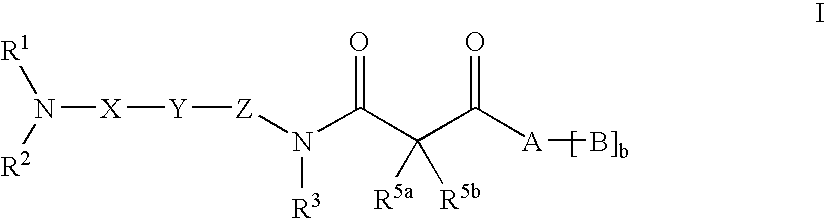
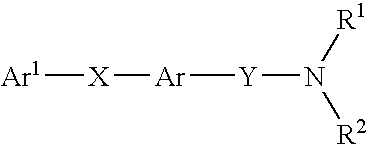Beta-ketoamide compounds with MCH antagonistic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i.1
Ethyl 3-biphenyl-4-yl-3-oxopropionate
[0314]
[0315] 14.7 g (75.0 mmol) of 4-acetylbiphenyl is dissolved in 150 mL of diethylcarbonate. Under protective gas, a total of 6.50 g (150 mmol) sodium hydride in oil (55%) is added batchwise at 0° C. The mixture is kept for 5 minutes at 0° C., then stirred for 2 hours at 80° C. After cooling, the mixture is poured onto water and extracted with methylene chloride; the organic phase is washed with water and finally dried over sodium sulfate. The solvent is eliminated, the residue is suspended in water and neutralized with 1N hydrochloric acid. The aqueous phase is extracted with diethyl ether, the organic phase is dried over sodium sulfate, and finally the solvent is eliminated. Lastly the residue is recrystallized from petroleum ether and the product is dried in vacuo at 50° C. Yield: 15.3 g (76% of theory); Rf value: 0.60 (silica gel, petroleum ether / ethyl acetate=5:2); m.p. 75° C.-77° C.; C17H16O3; EII mass spectrum: m / z=269 [M+H]+.
example ii.1
Ethyl 3-(4′-chlorobiphenyl-4-yl)-3-oxopropionate
[0316]
[0317] 4.29 g (32.5 mmol) of monoethyl malonate is dissolved in 100 mL of THF and 42.3 mL (67.7 mmol) of butyllithium solution (1.6N in hexane) is added dropwise at −60° C. The temperature is allowed to come up to −15° C., then the mixture is cooled again to −65° C. and 3.40 g (13.5 mmol) of 4′-chlorobiphenyl-4-carboxylic acid chloride (for preparation see Gazz. Chim. Ital. 1949, 79, 453.) in 30 mL of THF is added dropwise. The mixture is kept for 5 minutes at −65° C., then heated for 2 hours to ambient temperature. The mixture is poured onto 50 mL of 1N hydrochloric acid, extracted with 300 mL of diethyl ether, and the organic phase is washed with saturated sodium hydrogen carbonate solution and water and finally dried over sodium sulfate. The solvent is eliminated, the residue is recrystallized from petroleum ether and the product is dried in vacuo. Yield: 3.10 g (76% of theory); Rf value: 0.60 (silica gel, petroleum ether / eth...
example iii.1
3-chloro-4-[2-(4-methylpiperidin-1-yl)ethoxy]phenylamine
[0321]
III.a: 4-(2-bromoethoxy)-3-chloronitrobenzene
[0322] 36.6 mL (416 mmol) of 1,2-dibromoethane is dissolved in 200 mL of DMF and 11.5 g (83.3 mmol) of potassium carbonate is added. 7.20 g (41.6 mmol) of 2-chloro-4-nitrophenol in 40 mL of DMF is slowly added dropwise to this mixture. The mixture is stirred for 3 hours at ambient temperature. The solvent is eliminated, the residue is taken up in ethyl acetate and washed with saturated saline solution. The organic phase is dried over sodium sulfate. The solvent is eliminated and the residue is purified through a silica gel column with a gradient of petroleum ether / ethyl acetate (4:1 to 9:1). Yield: 7.9 g (68% of theory); Rf value: 0.55 (silica gel, petroleum ether / ethyl acetate=3:1); C8H7BrClNO3.
III.1.b: 3-chloro-4-[2-(4-methylpiperidin-1-yl)ethoxy]nitrobenzene
[0323] 7.80 g (27.8 mmol) of 4-(2-bromoethoxy)-3-chloronitrobenzene (III.1.a) and 10.1 mL (84.0 mmol) of 4-methylpi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com