Method for producing solid-lipid composite drug particles
a technology of solid-lipid composite drug particles and lipid-based drug particles, which is applied in the direction of drug compositions, solvent extraction, separation processes, etc., can solve the problems of limited use of drug-containing emulsions, limited liposome-based drug delivery systems, and limited biodegradable polymer nanoparticles, etc., and achieve low temperature processing and high drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
[0059] Preparation of Emulsions
[0060] Four organic solutions (Examples 1-4, respectively) were prepared by dissolving 5 percent of a lipid (GLUCIRE 50 / 13) and the amounts shown in Table 1 of either Indomethacin or Ketoprofen in chloroform (all amounts are specified as weight percents relative to the weight of the organic solvent). The resulting organic solutions were then dispersed in an aqueous 0.13% w / w of sodium glychocholate solution in the amounts shown in Table 1 in parts by weight relative to water to form coarse oil-in-water (O / W) emulsions. The resulting emulsions were then homogenized using a homogenizer commercially available from Microfluidics, Inc. (Newton, Mass.) at 16,000 p.s.i. in 3 passes.
TABLE 1Weight % ofWeight % of OrganicExampleDrugDrug in SolventSolution in Water1Indomethacin15102Indomethacin10303Indomethacin15304Ketoprofen1530
[0061] Precipitation of Nanoparticles
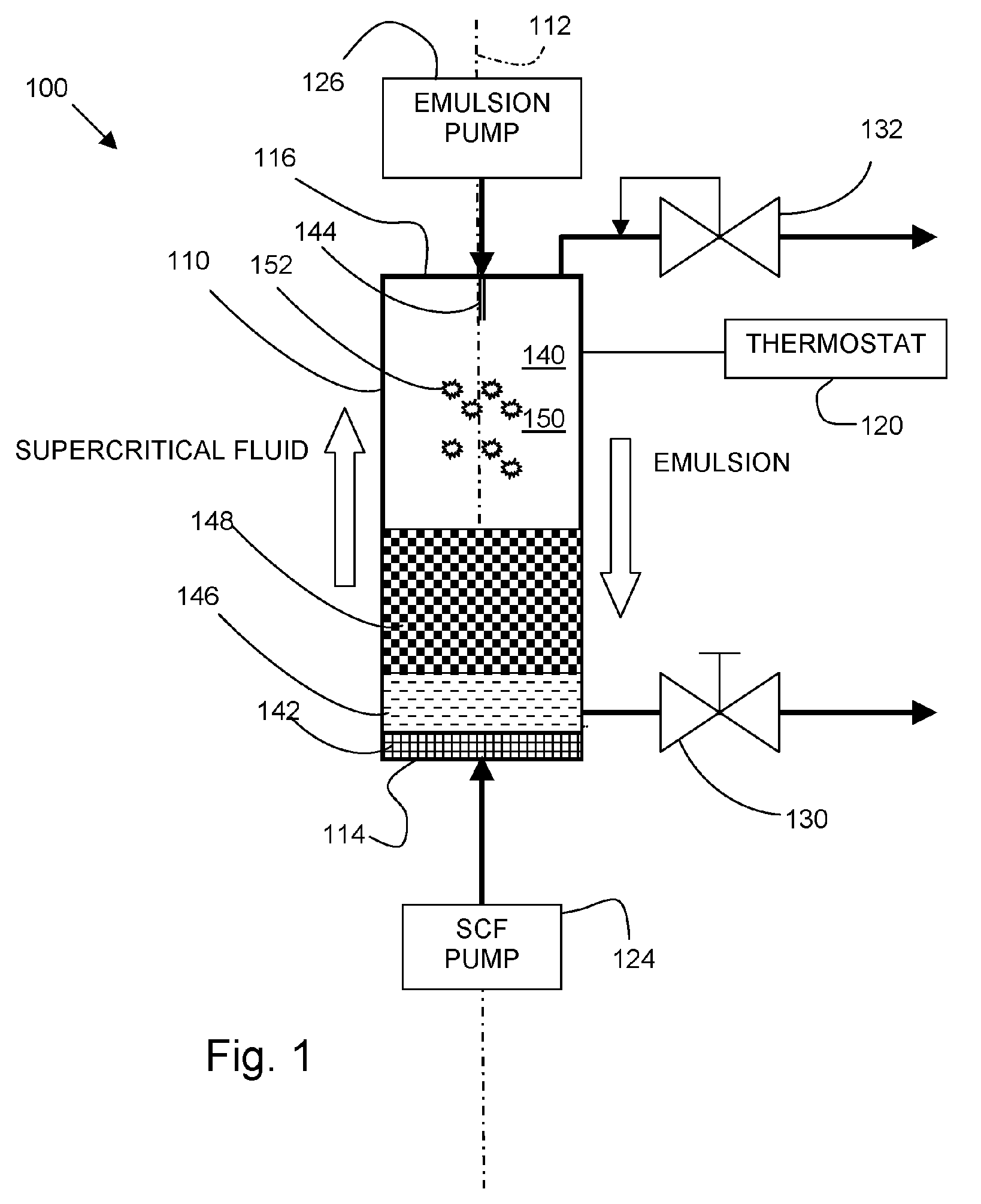
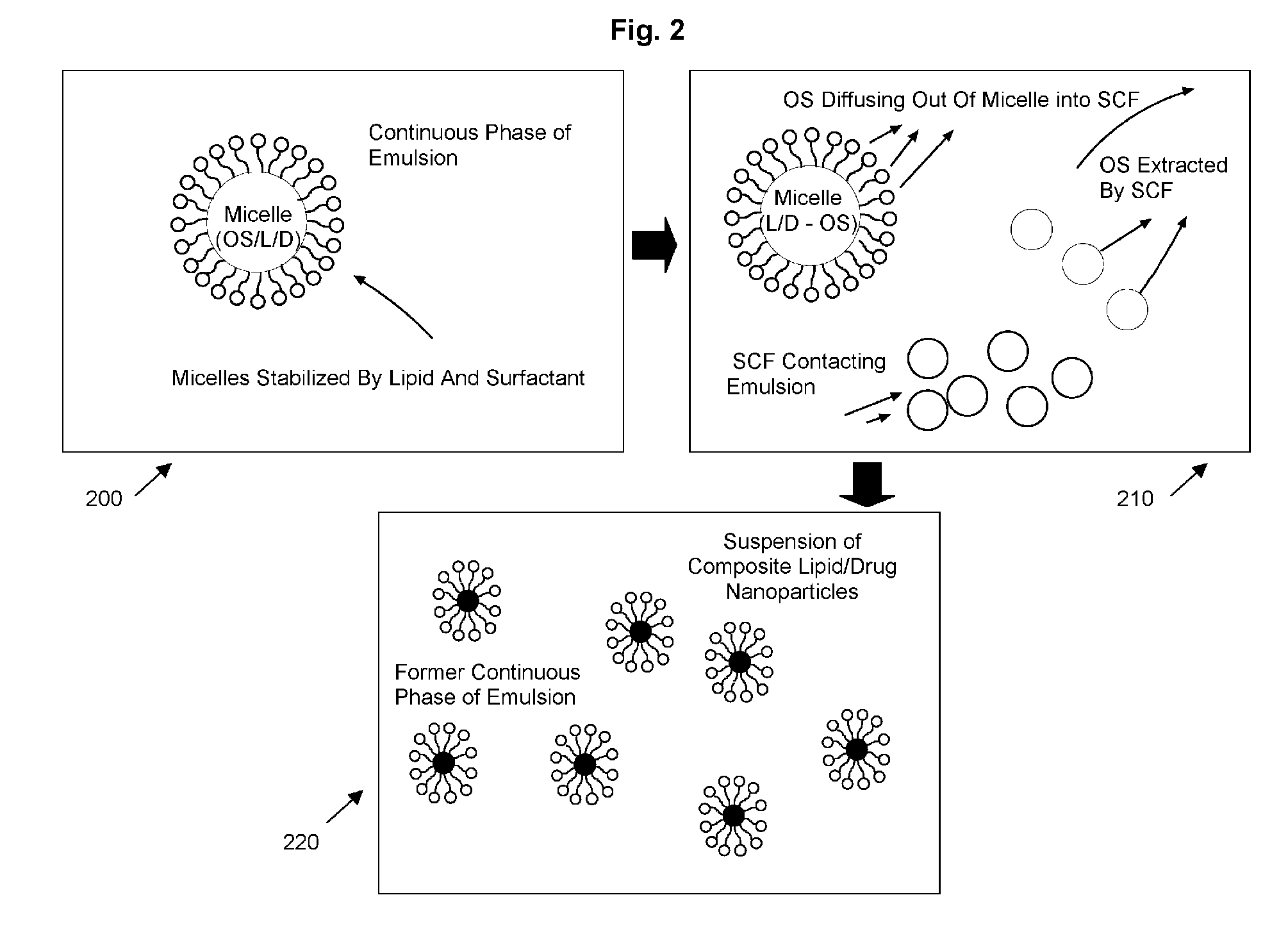
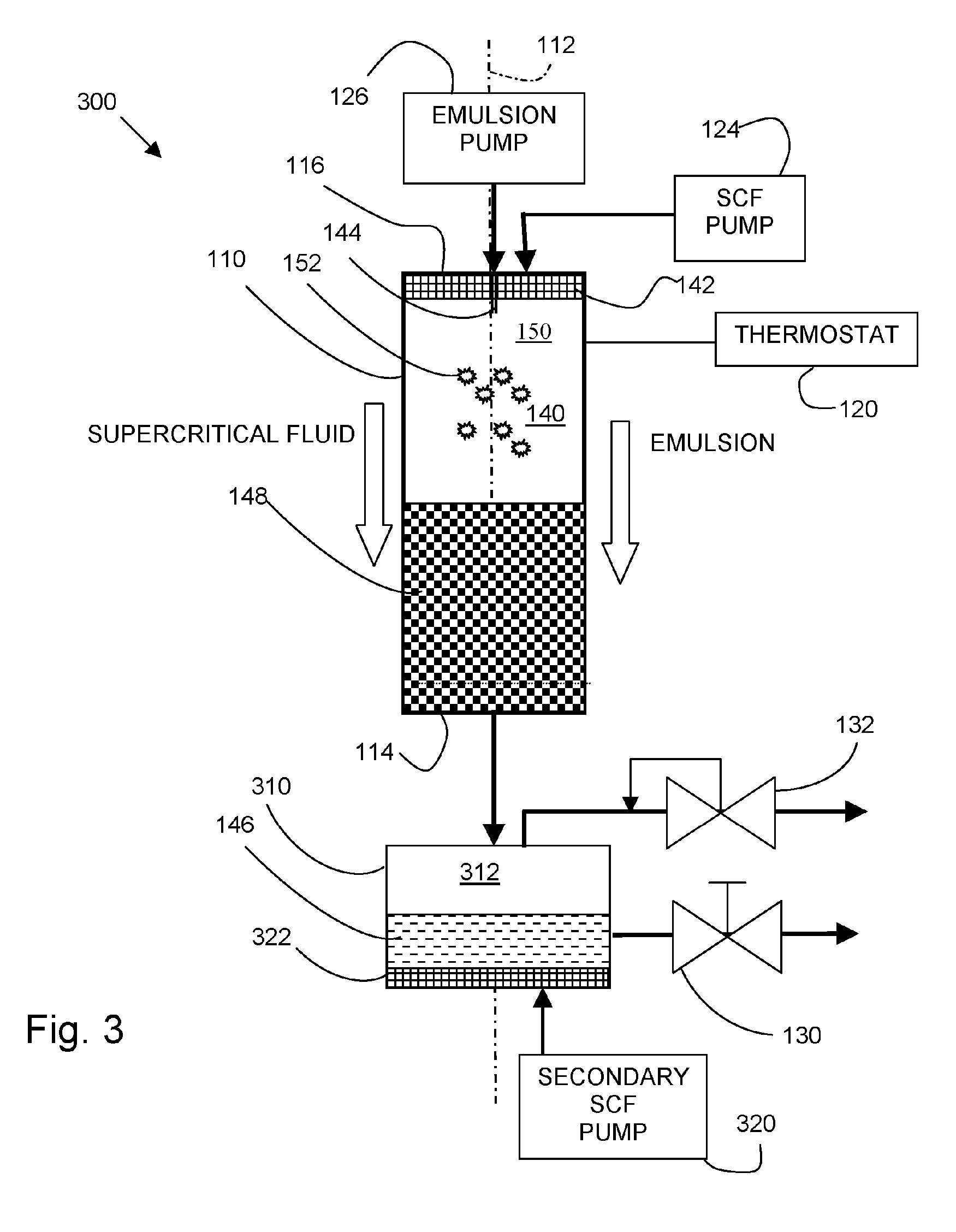
[0062] An apparatus such as shown in FIG. 1 was used to precipitate solid composite lipid / drug ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com