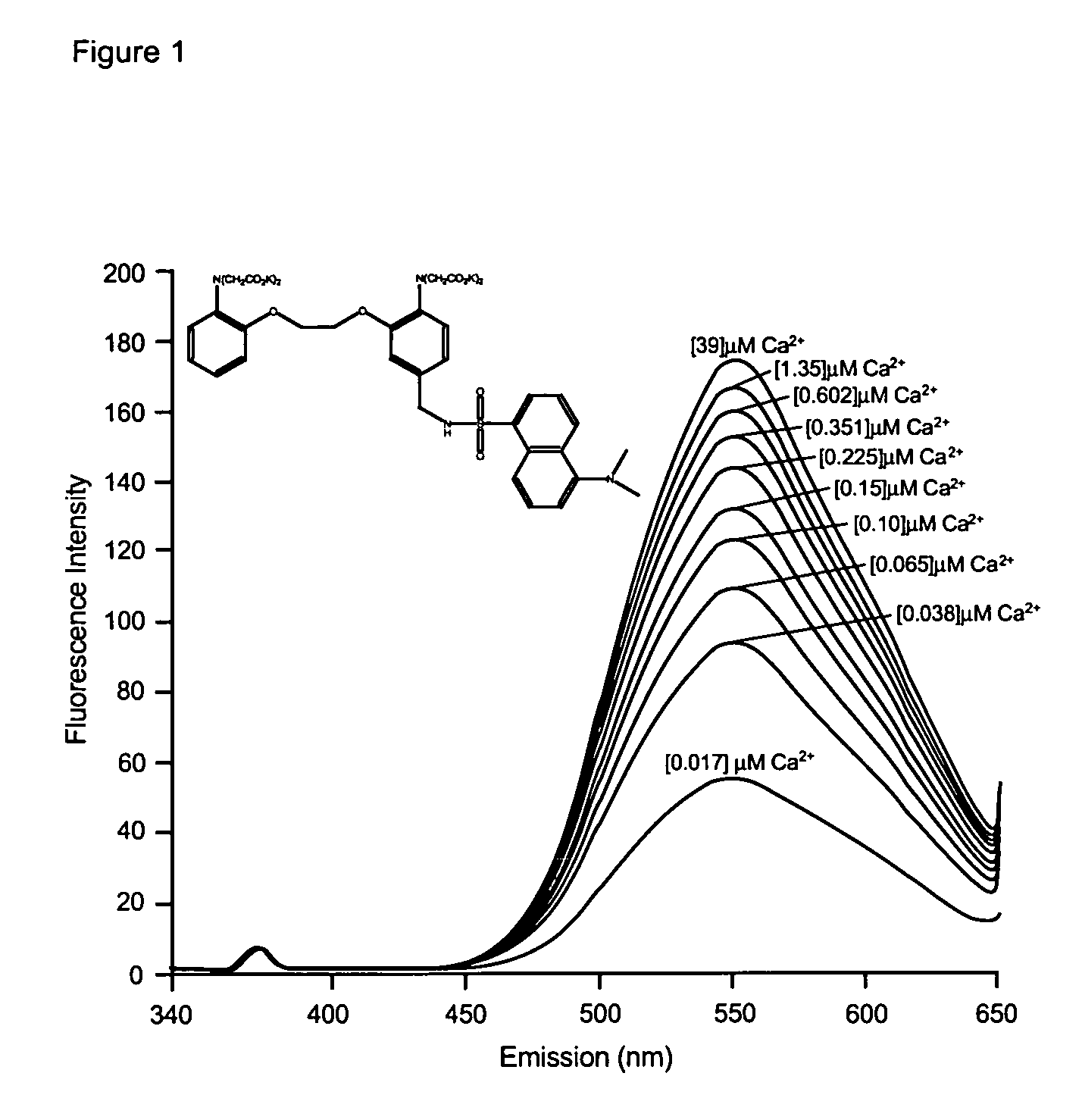
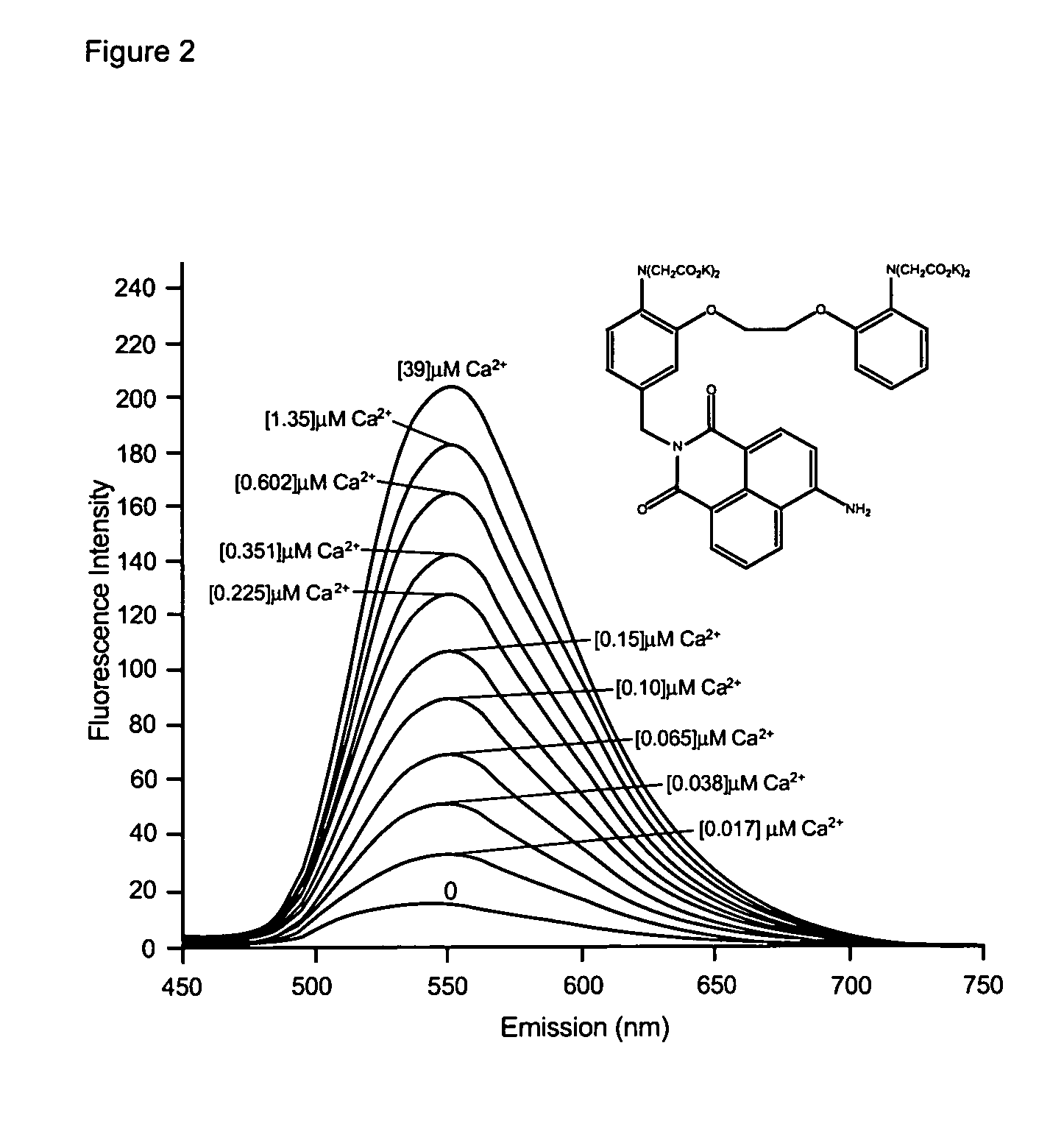
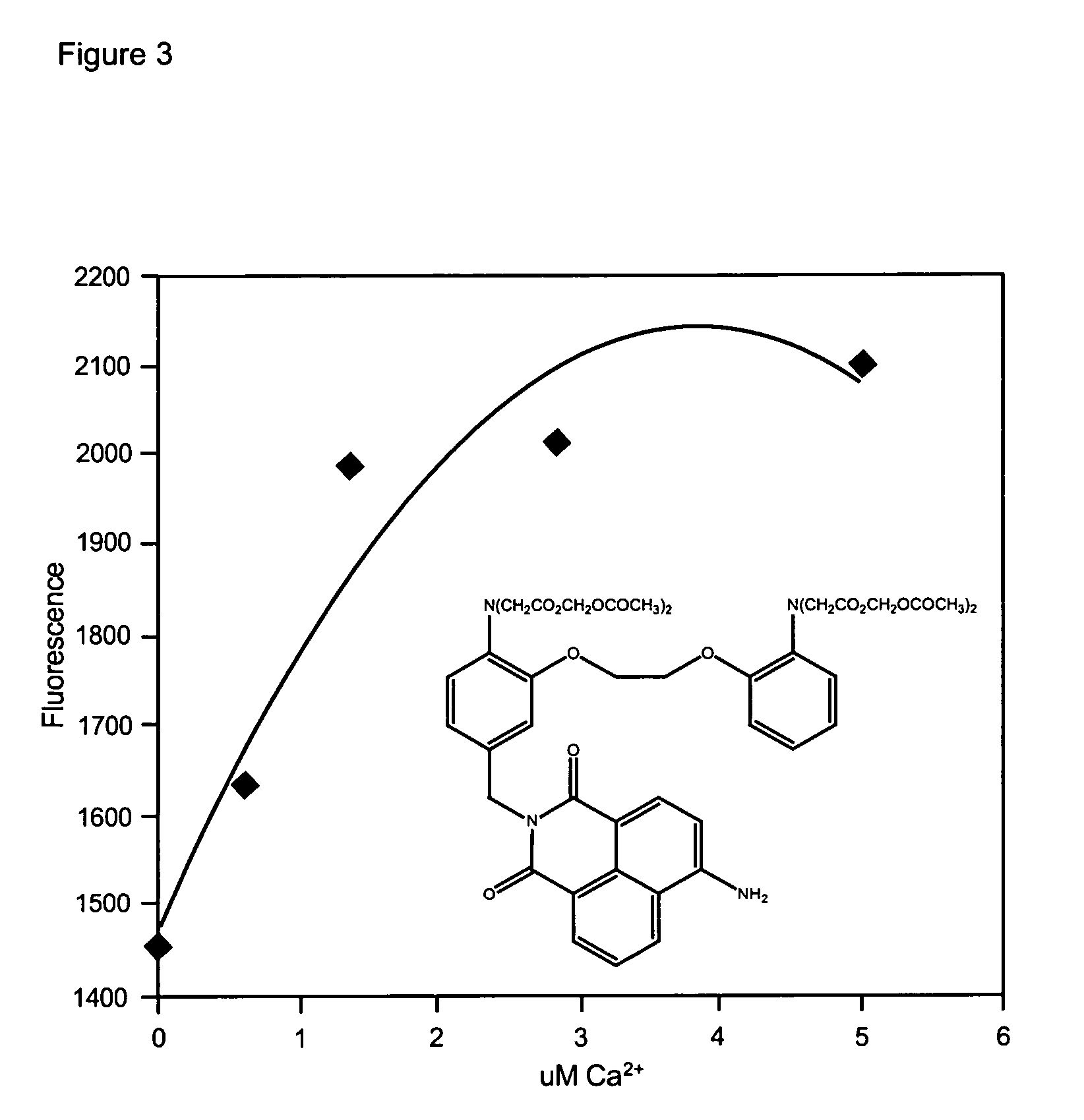
Fluorescent metal ion indicators with large stokes shift
a technology of metal ions and fluorescence, applied in the field of compositions and methods for the detection and isolation of metal ions, can solve the problems of limiting the effectiveness of metal ions and having a deleterious
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of 5-(Aminomethyl) BAPTA
[0183] To a stirred suspension of 5-formyl BAPTA 1 (5.60 g, 10 mmol) in EtOH (100 mL) was added a solution of hydroxylamine hydrochloride (1.40 g, 20 mmol) in H2O (5 mL) followed by 3N NaOAc (5 mL, 15 mmol). The mixture was stirred at 60° C. for 4 h, cooled to room temperature and evaporated. Water (200 mL) was added to the residue, the product filtered and washed with water (10×50 mL), dried in air, then in vacuo to give oxime 2, 5.37 g (91%) as an off-white solid. The product 2 does not require purification to use in the next step.
[0184] To a stirred solution of oxime 2 (4.71 g, 8 mmol) in acetic acid (80 mL), powdered Zn (2.62 g, 40 mmol) was added in one portion. The mixture was stirred for 6 h, diluted with CHCl3 (300 mL) and filtered from inorganic material. The filtrate was evaporated and the residue loaded onto a SiO2 column (3×30 cm bed, made in 5% MeOH and 1% AcOH in CHCl3). The product was eluted with a gradient of 5-20% MeOH in CHCl3 w...
example 2
Synthesis of 5-(Dansylaminomethyl) BAPTA derivatives
[0185] To a solution of amine 3 (160 mg, 0.28 mmol) in pyridine (3 mL), dry powdered dansyl chloride (98 mg, 0.36 mmol) was added in three portions within 5 min. The mixture was stirred for 2 h, then evaporated. The solid residue was dissolved in CHCl3 (100 mL), washed with H2O (100 mL), 1% AcOH (2×100 mL), and sat. NaCl (100 mL). Chloroform was evaporated and the residue was purified by preparative TLC on silica gel using 3% MeOH in CHCl3 as eluant to give 5-(dansylaminomethyl) BAPTA, tetramethyl ester 4, 60 mg (27%) as a white powder.
[0186] A mixture of tetramethyl ester 4 (60 mg, 0.074 mmol), MeOH (1 mL), dioxane (1 mL), H2O (1 mL), and 1N KOH (0.75 mL, 0.75 mmoL) was stirred for 16 h, then 0.2 N HCl added to achieve pH 9.5, and the mixture was evaporated. The residue was purified by column chromatography on Sephadex LH-20 (3.5×50 cm bed, made in H2O) using H2O as eluant to give 5-(dansylaminomethyl) BAPTA, tetrapotassium salt...
example 3
Synthesis of 5-(N-(5′-fluoresceinyl)aminomethyl) derivatives
[0188] To a stirred solution of amine 3 (115 mg, 0.2 mmol) and DIEA (0.17 mL, 1.0 mmol) in CH2Cl2 (5 mL) was added a solution of acyl chloride 8, prepared from the acid 7 (149 mg, 0.3 mmol) and oxalyl chloride (0.1 mL, 1.2 mmol). The mixture was stirred 16 h, then diluted with CHCl3 (100 mL), and washed with 1% AcOH (2×20 mL), sat. NaCl, filtered and evaporated. The residue was purified by preparative TLC on silica gel using 5% MeOH in CHCl3 as eluant to give tetramethyl ester 9, 71 mg (34%) as an off-white solid.
[0189] A mixture of tetramethyl ester 9 (50 mg, 0.047 mmol), MeOH (2 mL), dioxane (2 mL), and 1N KOH (0.5 mL, 0.5 mmoL) was stirred for 16 h, then 0.2 N HCl added to pH 9.0, and the mixture was evaporated. The residue was purified by column chromatography on Sephadex LH-20 (6×70 cm bed, made in H2O) using H2O as eluant to give hexapotassium salt 10, 39 mg (72%) as a yellow solid (after lyophilization).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Nanoscale particle size | aaaaa | aaaaa |
| Nanoscale particle size | aaaaa | aaaaa |
| Nanoscale particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com