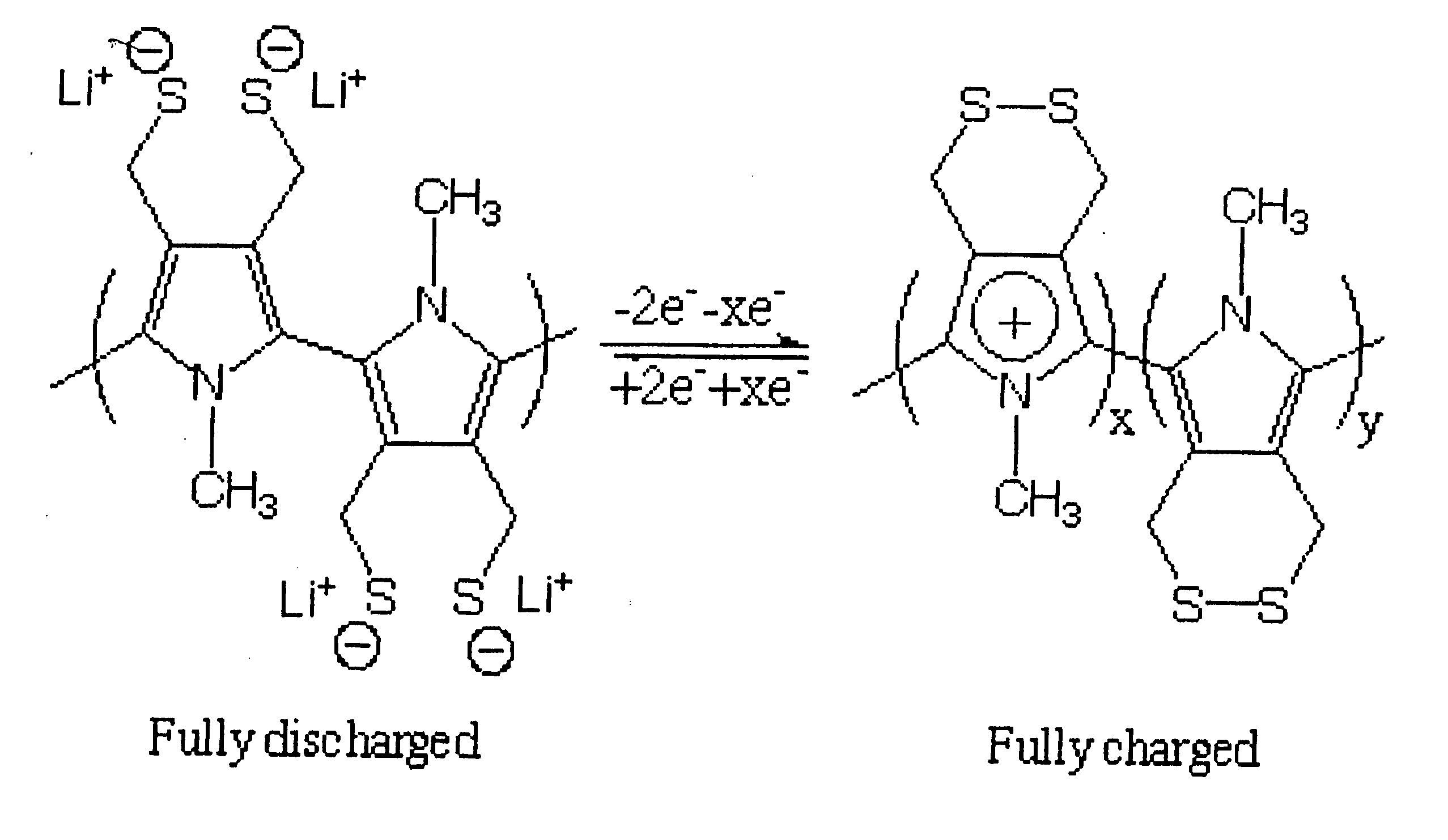
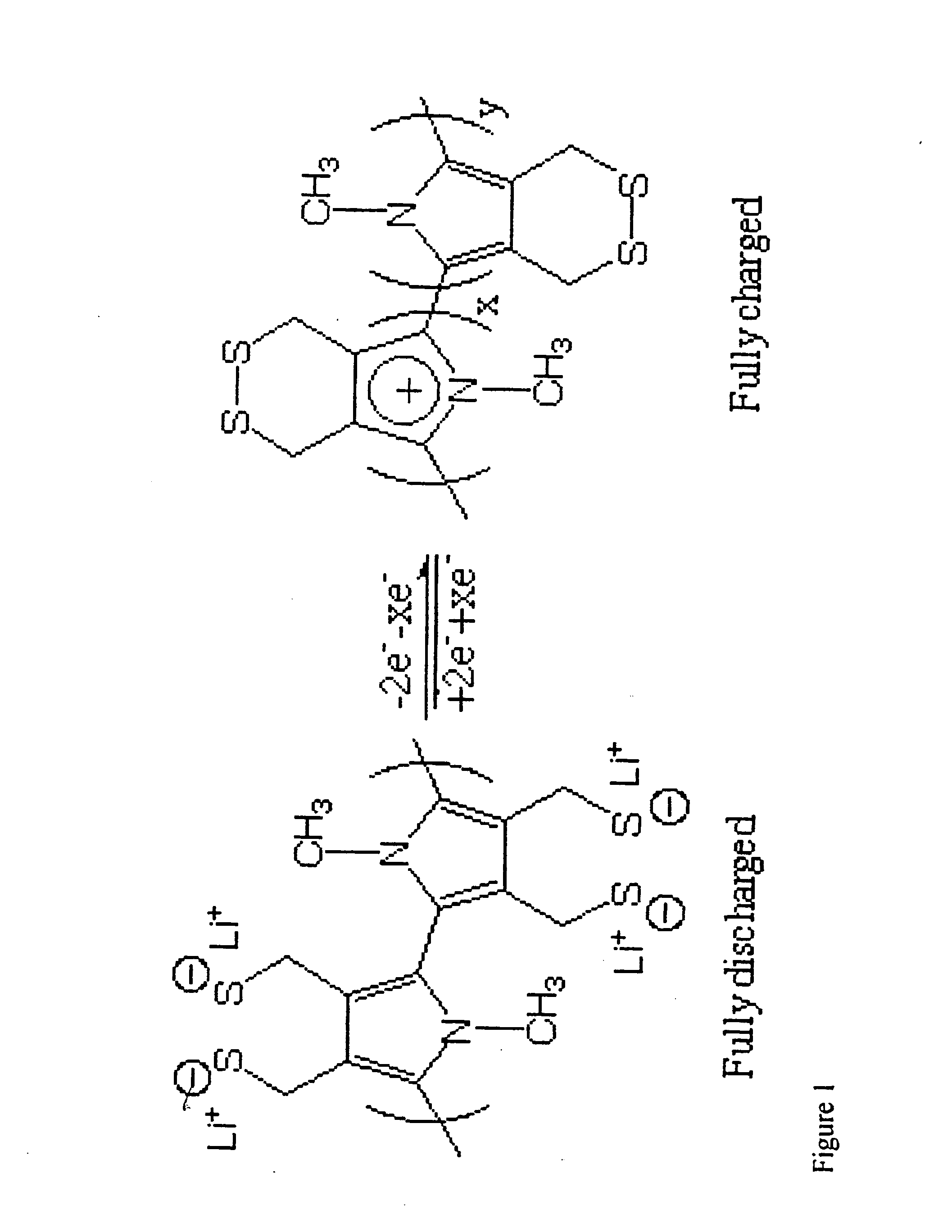
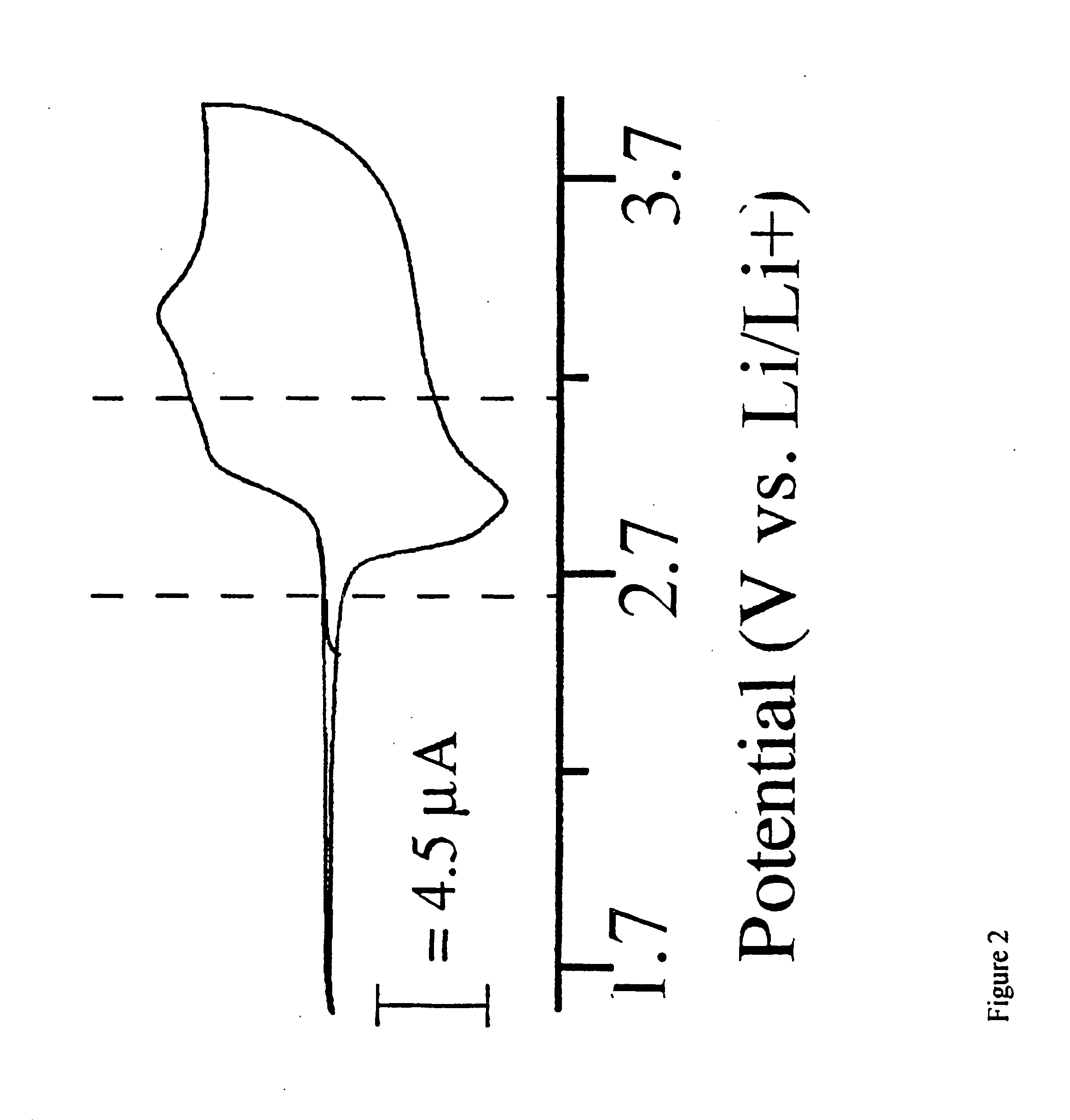
Single component sulfur-based cathodes for lithium and lithium-ion batteries
a sulfur-based cathode and lithium-ion battery technology, applied in the direction of non-metal conductors, cell components, conductors, etc., can solve the problems of less desirable materials and poor battery performance, and achieve the effect of attractive cathode use, wide potential conductivity window, and attractive cathode us
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0068] The first attempted, unsuccessful, synthesis involved Cu(l) assisted nucleophilic displacement of the bromine groups of 3,4-dibromothiophene with 1,2-dithiane-4,5-diol to form the desired cyclic ester (Scheme 1).
Experimental:
[0069] 3,4-(1,2-dithiane-4,5-diol) Thiophene. Pyridine (5 mL) was added to 1,2-dithiane-4,5-diol (0.27 g, 1.80 mmol). CuI (0.01 g, 0.07 mmol) and KOtBu (0.06 g, 0.54 mmol) to produce a yellow solution which turned green after stirring at ambient temperature over a period of 1 h, 3,4-dibromothiophene (0.09 g, 0.36 mmol) was then added to the reaction mixture which was heated at 100° C. for 24 h. A distillation was then done to remove pyridine. The remaining solution was dissolved in 0.05 M EDTA to remove complexed copper. An extraction was done with ethyl acetate. The organic, ethyl acetate, layer was concentrated under vacuum. This procedure did NOT result in the desired compound, the actual identity of the remaining solution was not confirmed.
[0070] ...
example 2
[0076] The use of 3,4-dihydroxythiophene is also necessary in the synthesis of the second desired monomeric component shown in FIG. 2 and will be synthsized as described above. Scheme 4 shows the mechanism of formation of the compound shown in FIG. 2. The reaction of 3,4-dihydroxythiophene with 2,2-dimehtyl-1,3-dioxolane-4-methanol in the presence of a base, followed by H3O+ addition, resulted in the formation of compound K. Alternatively, this is also formed by the addition of 2 equivalents of allyl bromide to 3,4-dihydroxythiophene in the presence of a base. The resulting product, J, was treated with catalytic amounts of OsO4 and sodium periodate to produce compound K. Once compound K was obtained it was treated with PBr3 or tosyl chloride to form L, where LG=Br or OTs. Compound L can also be produced by the addition of 1,2,3-tribromopropane to 3,4-dihydroxythiophene. Treatment of L with potassium thioacetate resulted in formation of M. Polymerization of this material was done in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| charge capacity | aaaaa | aaaaa |
| electronic conductivity | aaaaa | aaaaa |
| charge capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com