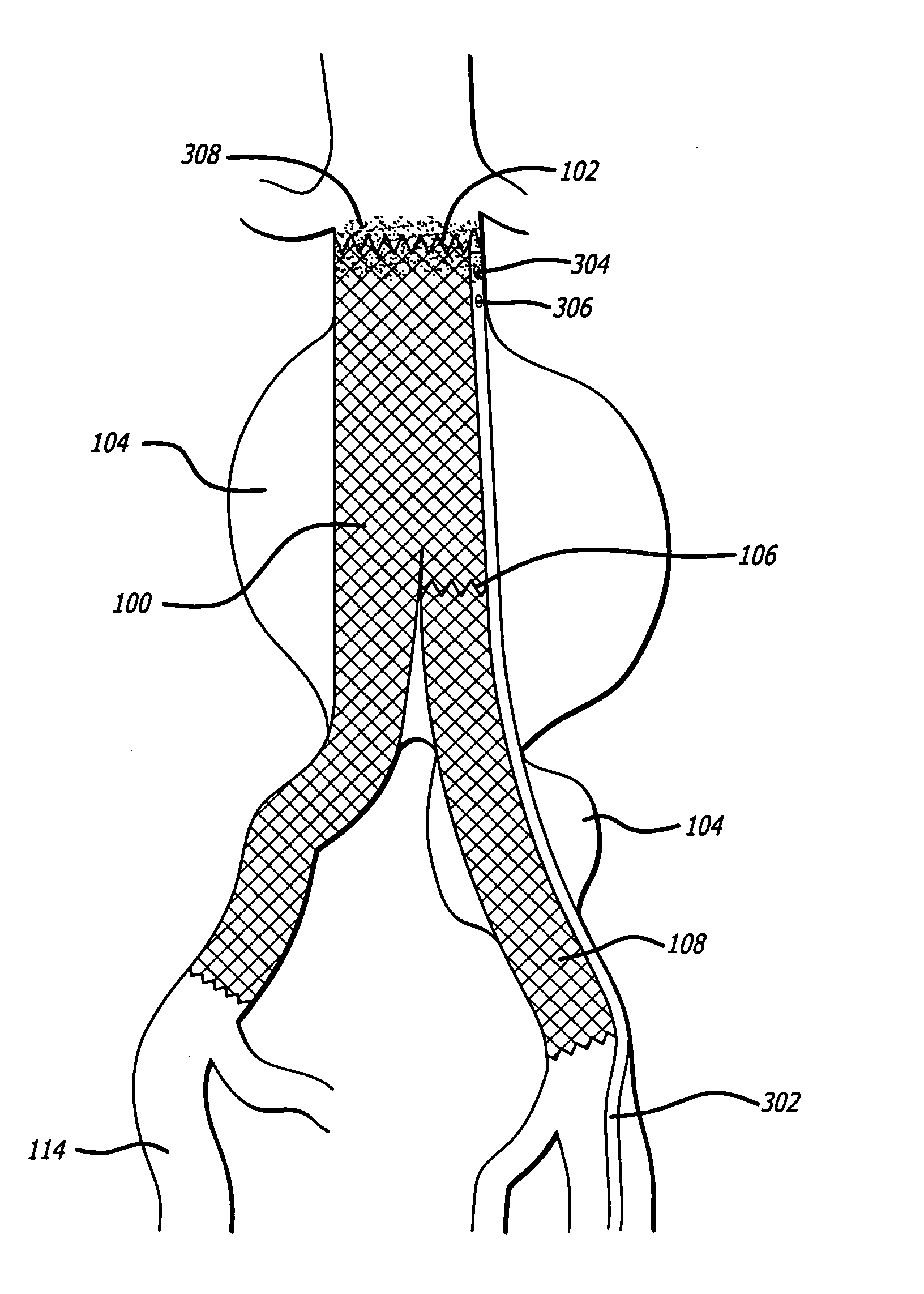
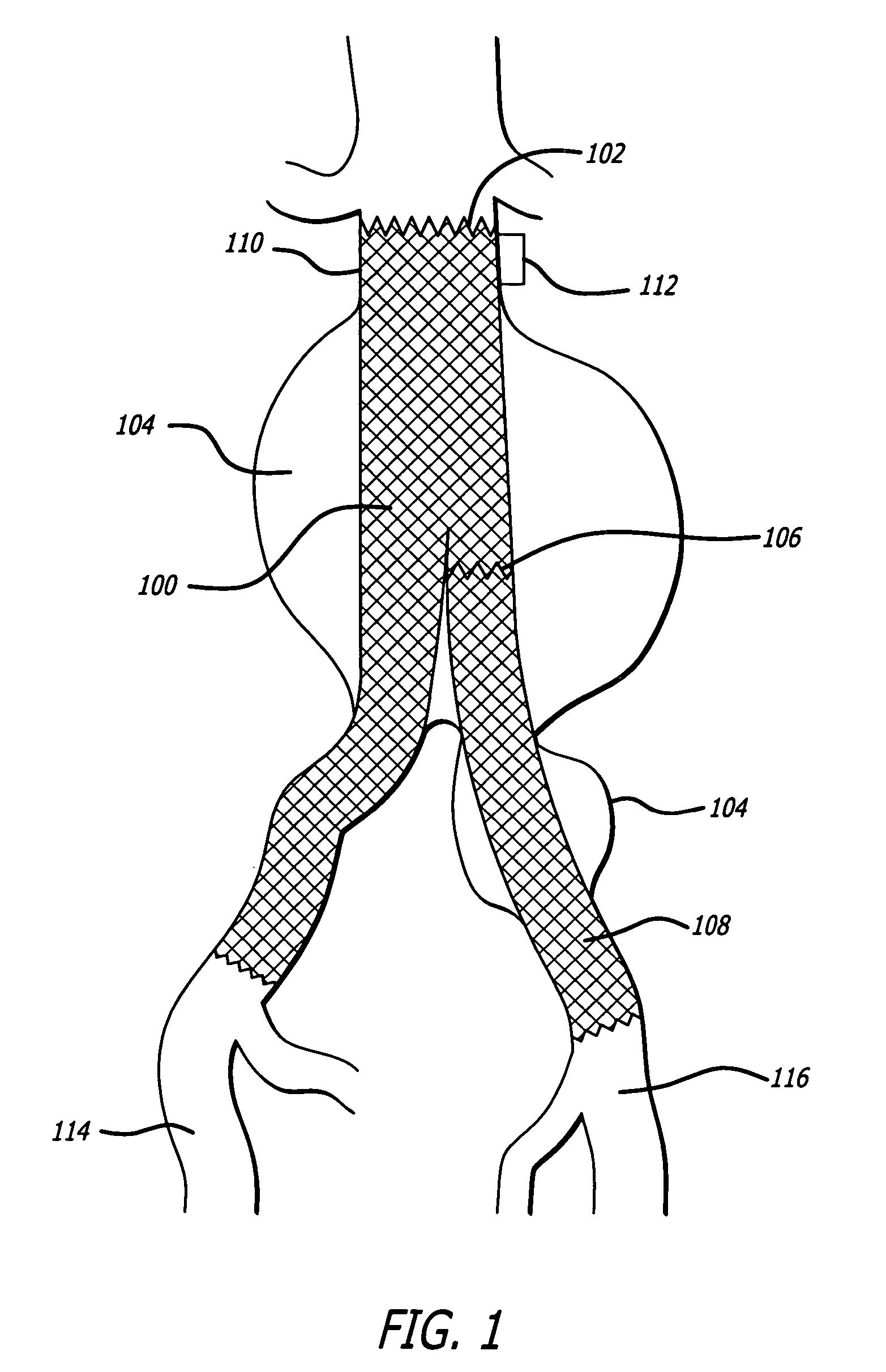
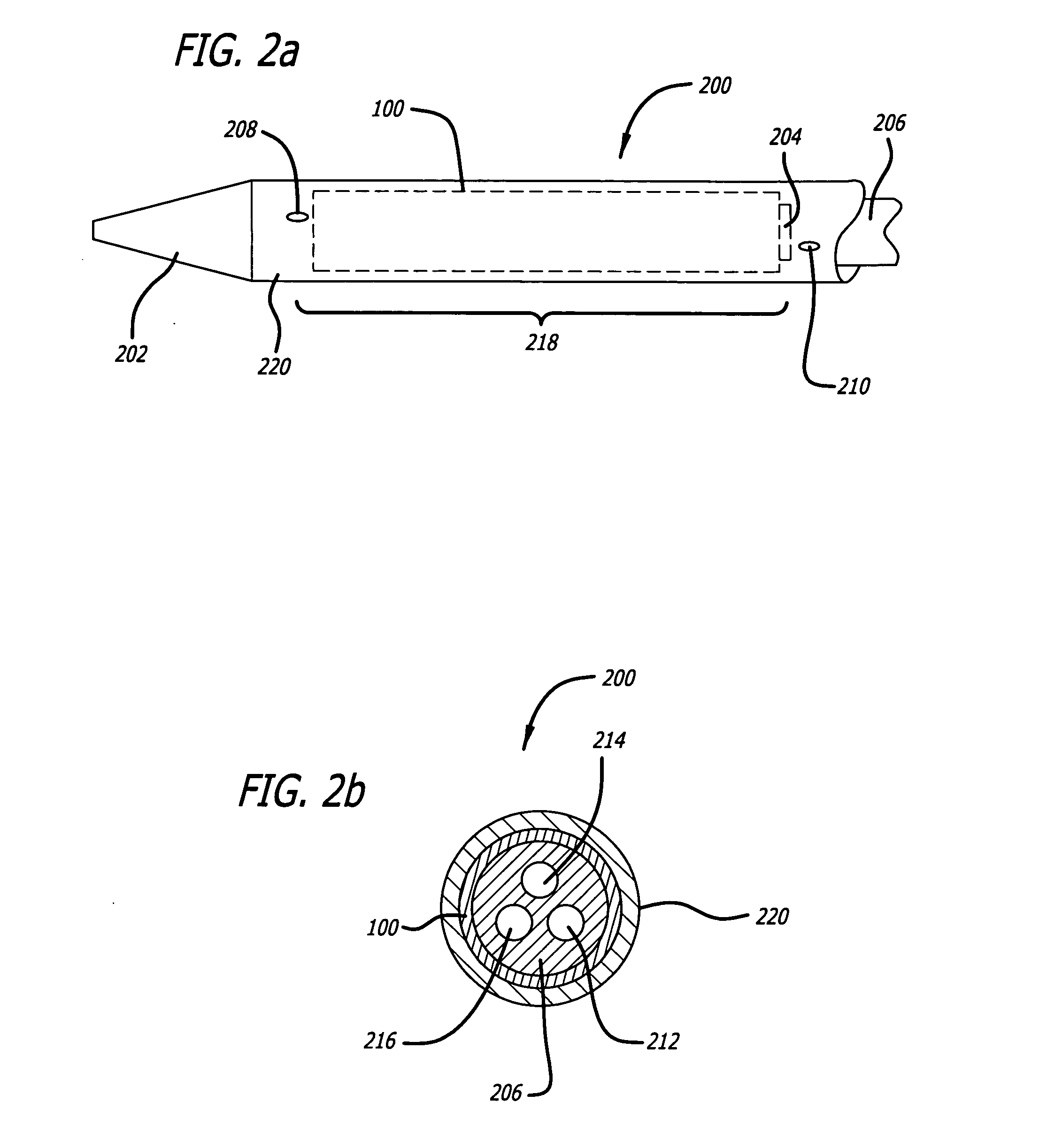
Autologous platelet gel on a stent graft
a technology stent, which is applied in the field of autologous platelet gel on stent graft, can solve the problems of approximately 14,000 u.s. deaths per year, aneurysms are asymptomatic, and aneurysm deaths are suspected of being underreported, so as to promote endothelialization of stent graft, promote stent graft strengthening and fixation, and enhance the proliferation of smooth muscl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Properties of Platelet Rich Plasma
[0066] Aliquots of human peripheral blood (30-60 mL) are passed through the Magellan™ Autologous Platelet Separation System (the Magellan™ system) and the concentrated, platelet-rich plasma fraction (PRP) assayed for platelets (PLT), white blood cells (WBC) and hematocrit (Hct) (Table 1). The Magellan™ system concentrated platelets and white blood cells six-fold and three-fold respectively.
TABLE 1Blood cell yields after passing through the Magellan ™ system.Mean ± SDn = 19Initial BloodPRPYieldPLT (× 1000 / μL)220.03 ± 48.581344.89 ± 302.006.14 ± 0.73WBC (× 1000 μ / L) 5.43 ± 1.4317.04 ± 7.013.12 ± 0.90Hct (%)38.47 ± 2.95 6.81 ± 1.59
Cell Yield = cell count in PRP / cell count in initial blood = [times baseline]
[0067] Additionally, PRP was assayed for levels of the endogenous growth factors platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-β), basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), and ...
example 2
Autologous Platelet Gel Generation
[0068] Autologous Platelet Gel (APG) is generated from the PRP fraction produced in the Magellan system by adding thrombin and calcium to activate the fibrinogen present in the PRP. For each approximately 7-8 mL of PRP, approximately 5000 units of thrombin in 5 mL 10% calcium chloride are required for activation. The APG is formed immediately upon mixing of the activator solution with the PRP. The concentration of thrombin can be varied from approximately 1-1,000 U / mL, depending on the speed required for setting to occur. The lower concentrations of thrombin will provide slower gelling times.
example 3
Effects of APG on Cell Proliferation
[0069] A series of in vitro experiments were conducted evaluating the effect of released factors from APG on the proliferation of the human microvascular endothelial cells, human coronary artery smooth muscle cells and human dermal fibroblasts. Primary cell cultures of the three cell types were established according to protocols well known to those skilled in the art of cell culture. For each cell type, three culture conditions were evaluated. For APG cultures, APG was added to cells in basal medium. A second group of cells were cultured in growth medium. Growth medium is the standard culture medium for the cell type and contains optimal growth factors and supplements. The control cultures contain cells cultured only in basal medium which contains no growth factors.
[0070] Autologous platelet gel had a significant growth effect on human coronary artery smooth muscle cells after five days of culture (FIG. 7), human microvascular endothelial cells ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com