New type of catalytic materials based on active metal-hydrogen-electronegative element complexes for hydrogen transfer
a technology of electronegative element and active metal, which is applied in the direction of organic compound/hydride/coordination complex catalyst, inorganic chemistry, fuel cell, etc., can solve the problems of increasing the cost of metal, improving the efficiency, and not fully understanding the role of the suppor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Ti-Based Catalyst Prepared by Reaction with Methanol
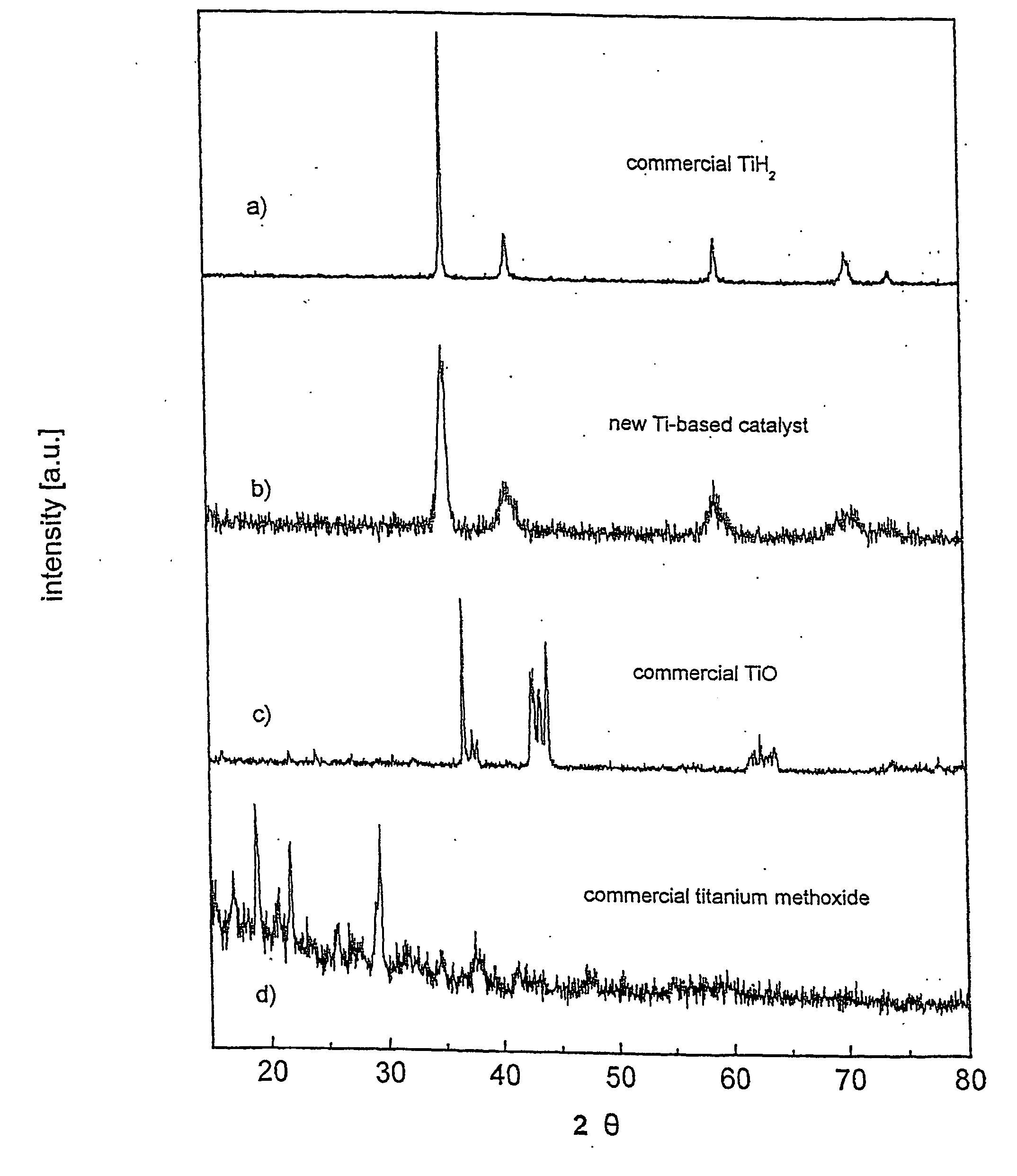
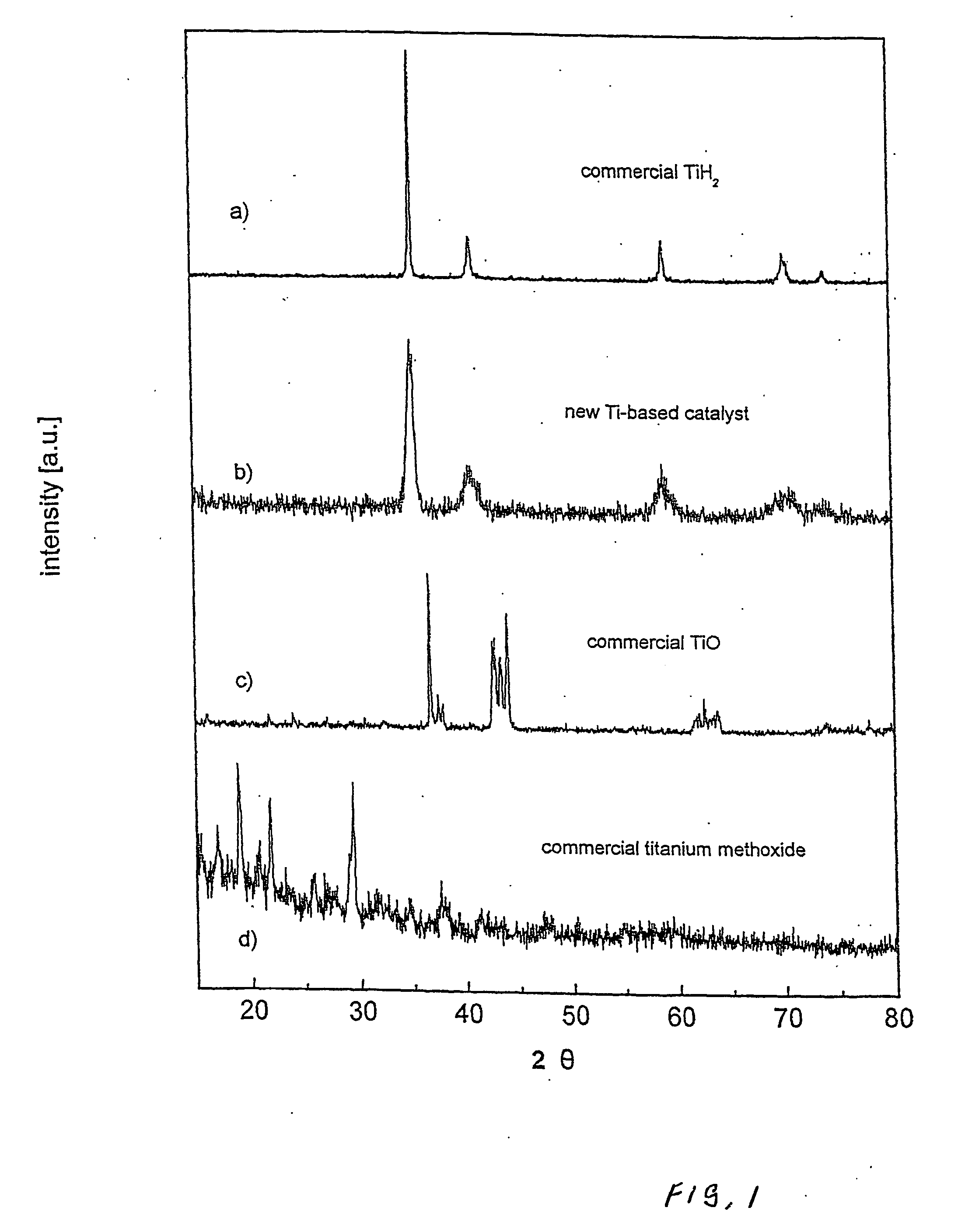
[0084] A Ti-based catalyst was produced both from titanium powder and titanium hydride. Both methods gave equally good catalytic capability of the resulting catalyst so long as deleterious oxidation of titanium was prevented.
[0085] Commercial titanium hydride (TiH2) was used as a starting material, and was purchased from Aldrich (purity 98%, powder −325 mesh). X-ray diffraction pattern of this hydride was created using a Bruker D8 Discover X-ray diffraction system (as was the case for all X-ray diffraction results discussed herein) and is shown in FIG. 1a. The X-ray diffraction pattern exhibits a characteristic set of Bragg's reflections consistent with the International Centre for Diffraction Data database PDF-2, card number 65-0934.
[0086] One gram of titanium hydride was loaded into a stainless steel vial together with approximately 1 ml of methanol (methyl alcohol HPLC grade 99.9%) and stainless steel balls, giving a ball-to-...
example 2
A Zr-Based Catalyst
[0096] Zirconium-based catalysts according to the invention can be produced from both zirconium and zirconium hydride. In general, it involves formation of the Zr—H atomic configuration, complemented by introduction of the electronegative element. For example, the electronegative element can be derived from a liquid such as water or alcohol, or from, for example, metal oxides. Similar to the above examples using titanium, a variety of processes can be effectively applied in the preparation of the Zr-based catalysts.
[0097] In the current example, zirconium powder was purchased from Alfa Aesar (with purity 95+%, average powder size 2-3 micron, packaged in water). The disadvantage of metallic zirconium is that it is very sensitive to oxidation. Since normally zirconium does not react with water, packaging in water is the most common method of protecting Zr from deterioration in air. Although in some cases water can be used as a reagent in the process of preparation...
example 3
Use of Metal Oxides in Catalyst Formation
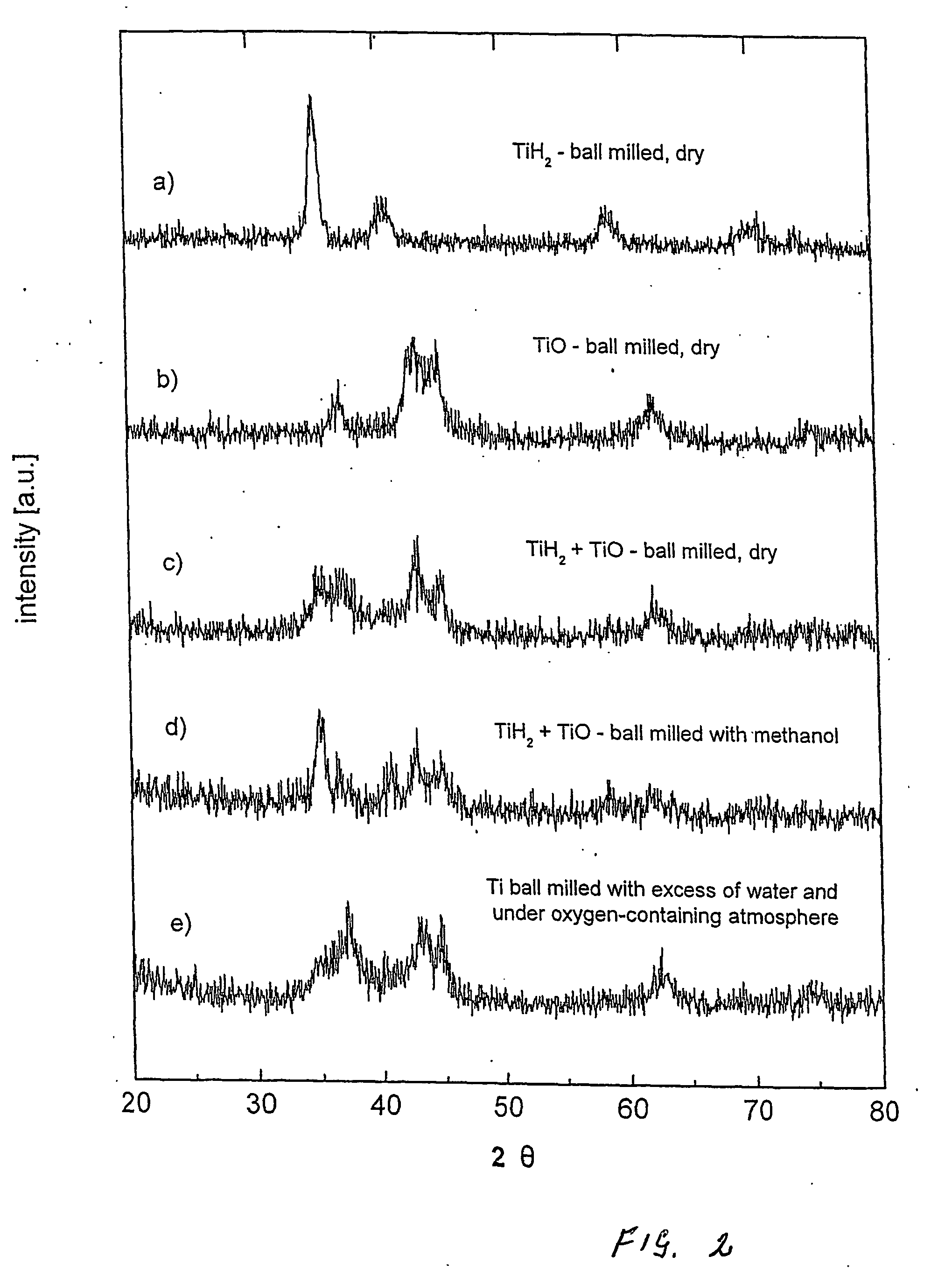
[0106] The following experiments describe examples of various methods and compositions to produce new catalysts with outstanding catalytic ability. One important variation is the use of metal oxides as donors of the electronegative element. The advantage of the use of easily reducing oxides in combination with liquids (such as water and alcohol) in the ball milling process, is that the contribution of the electronegative element is in this way more easily controlled, through a “self-adjusting” mechanism of partial (or full) reduction of the oxide.
(a) Uses of Metal Oxides to Form Zr-Based Catalysts
[0107] Another zirconium-based catalyst was produced from zirconium hydride (ZrH2) and copper oxide (CuO), in a process of ball milling with a mixture of water and methanol. 400 mg of zirconium hydride ZrH2 (Alfa Aesar, purity 99.7%, c) indicates that at least a portion of CuO was reduced during the milling process, and Bragg's reflection charact...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com