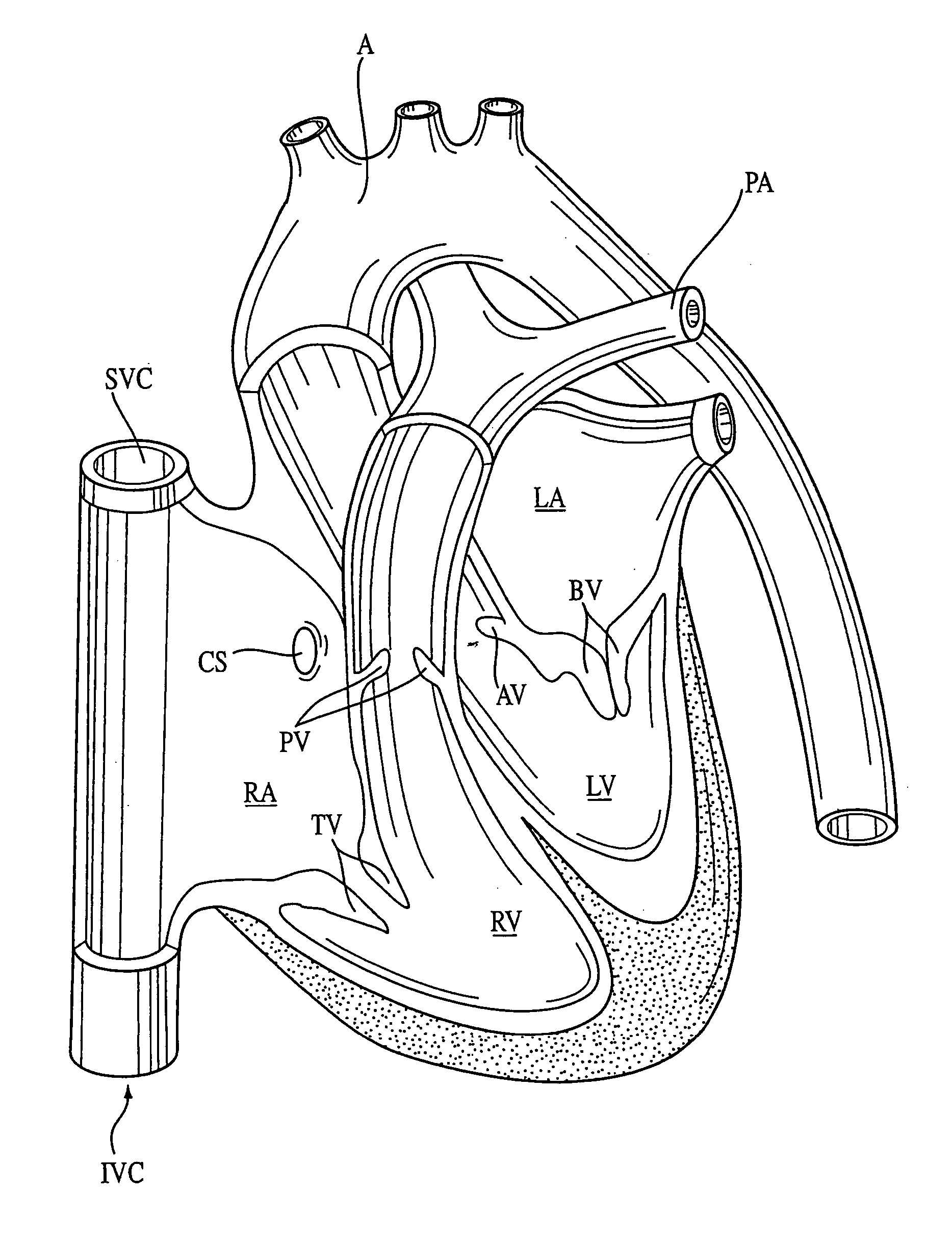
Compositions, kits, methods, and apparatus for transvascular delivery of a composition to an extravascular tissue of a mammal
a technology of composition and mammalian tissue, applied in the field of gene therapy and cardiac therapy, can solve the problems of high psychological toll of patients who undergo surgery, inability to readily access the circulating virus in a mammal, and high cos
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
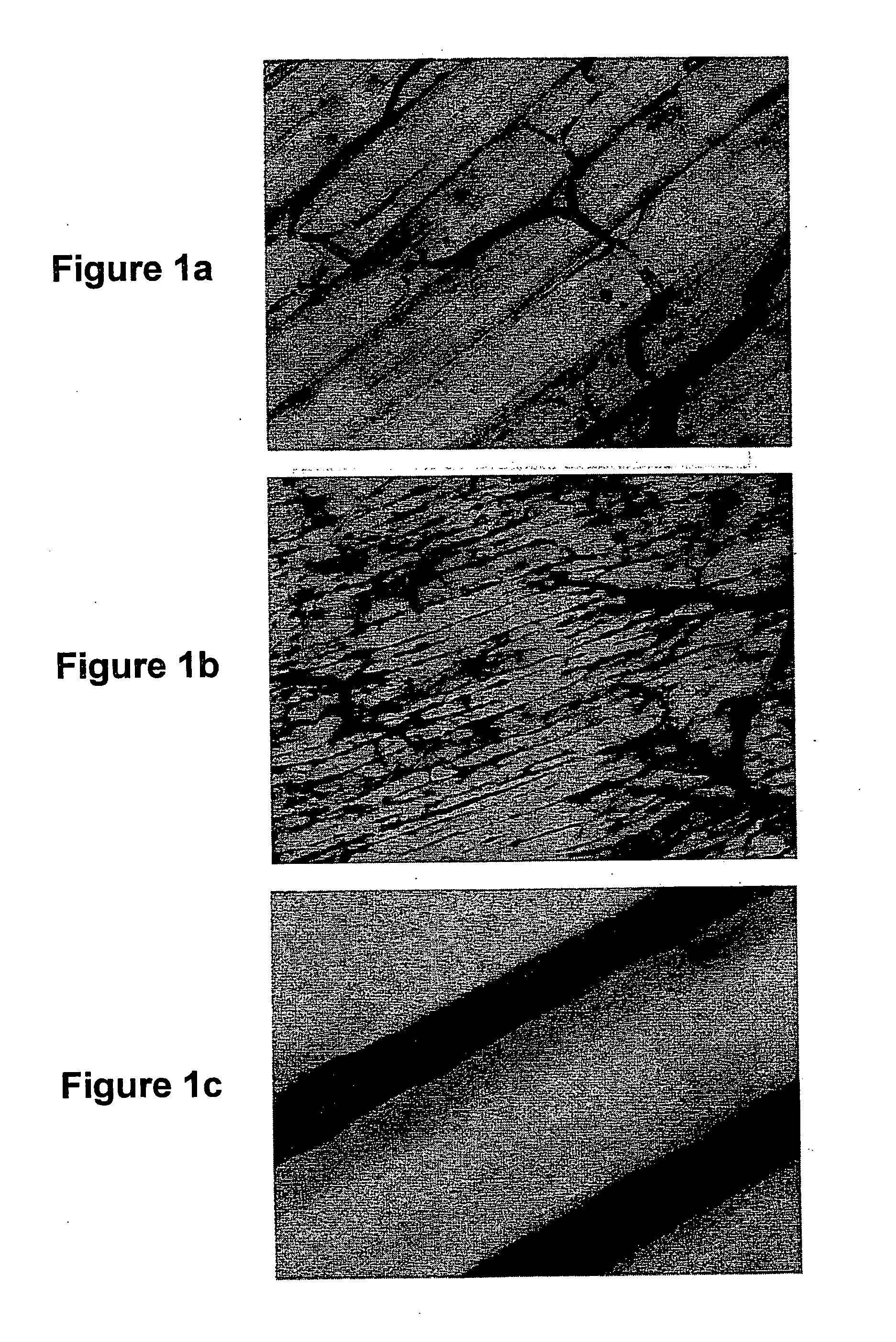
[0217] Convectional Transport of Adenovirus Across the Microvascular Barrier Promotes Systemic Gene Transfer
[0218] Data which supports the hypothesis that transient hepatic inflow occlusion minimizes unwanted viral sequestration are now presented. The experimental Pringle maneuver was performed on rats which were administered recombinant adenovirus into the central circulation. This resulted in greatly reduced hepatic staining intensity relative to control mammals on which the Pringle maneuver was not performed.
[0219] The methods used in the experiments presented in this Example are now described.
[0220] Construction and amplification of the E1, E3 deleted recombinant adenovirus vector designated AdCMVlacZ has been described (Kozarsky et al., 1993, Som. Cell Molec. Genet. 5:449-458). Recombinant adenovirus was administered to rats using a total dose of approximately 109 particles per gram of rat body weight. A frozen aliquot of the virus stock, which comprised 10% (v / v) glycerol, ...
example 2
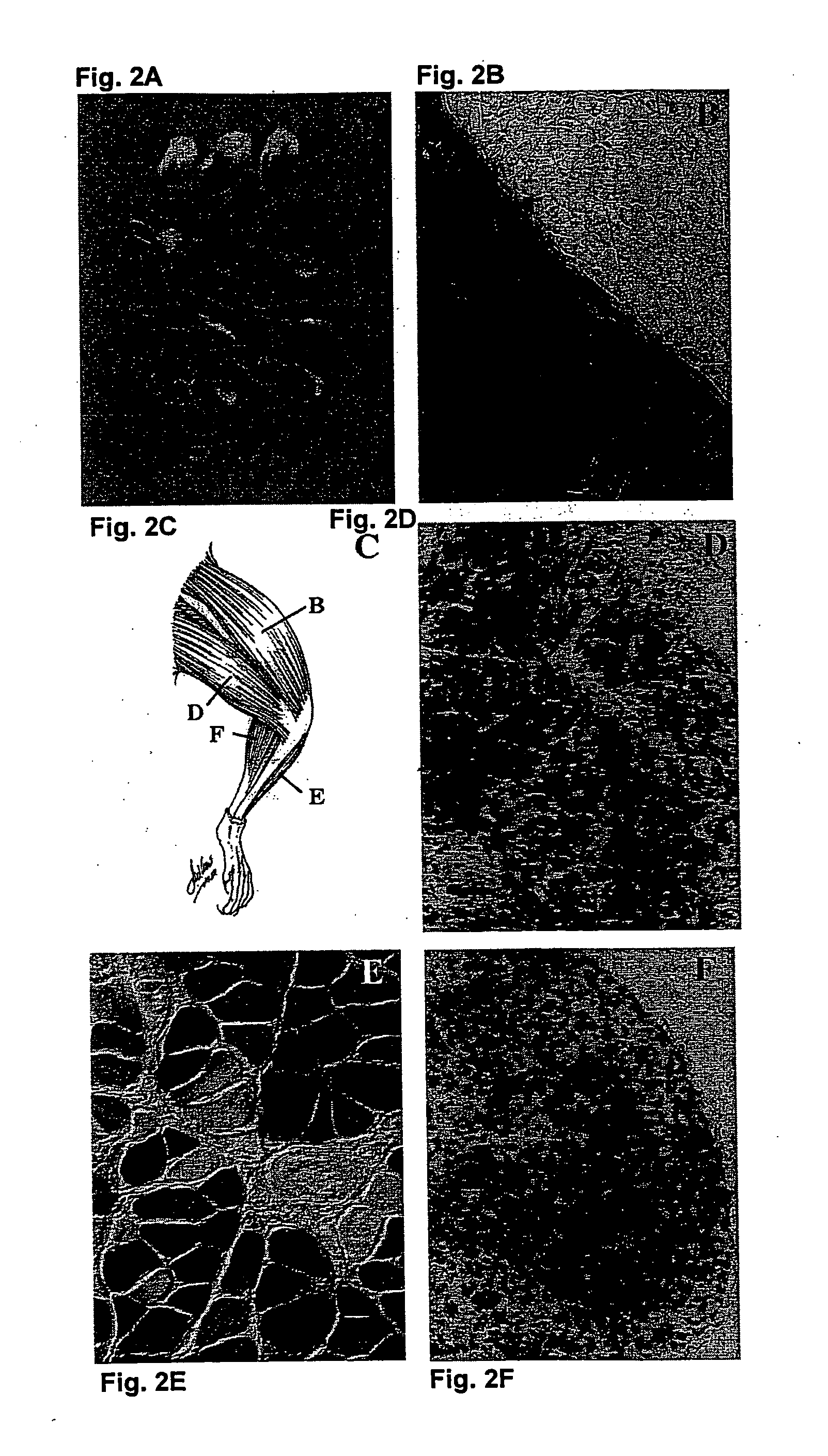
[0255] Additional Compounds Useful for Enhancing Microvascular Permeability
[0256] Compounds which may also function to enhance microvascular permeability in addition to histamine and papaverine, include, but are not limited to, platelet activating factor, serotonin, bradykinin and nitroprusside. Capillary permeability induced following administration of these compounds may be assessed by quantifying the uptake of fluorescently labeled 70- to 100-nanometer-diameter dextran particles.
[0257] Given the data which has been described, the invention may be extended as follows.
[0258] Systemic gene transfer may be accomplished in large mammals, including humans using a combination of inflammatory and vasodilatory agents provided extracorporeal support is in place.
[0259] Provision of adequate circulatory support and oxygenation depends directly on the implications of allometric scaling, i.e., the mathematical relationship which governs the organ function of mammals of different size. Each...
example 3
[0263] Extracorporeal Circulatory Support and Blood Oxygenation in Sheep
[0264] The experiments described in this Example were performed to confirm that the circulatory system of a mammal larger than a mouse or a rat can be supported extracorporeally under the conditions described herein for delivery of a macromolecular assembly such as an adenovirus vector. Sheep were subjected to cardiopulmonary bypass, and were then administered either 3.75 or 7.5 milligrams per kilogram body weight of papaverine and either 25 micrograms per kilogram body weight or 125 milligrams per kilogram body weight of histamine. Relevant physiological characteristics of the sheep were monitored in real time.
[0265] The materials and methods used in the experiments presented in this Example are now described.
[0266] Surgical Procedure
[0267] The extracorporeal circulation support system described herein was designed to permit the mammal to tolerate massive fluid exudation under the influence of histamine and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Stokes radius | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com