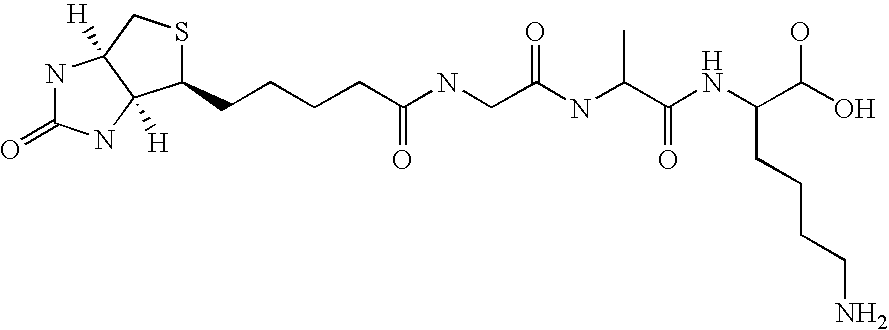
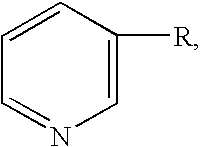
Patents
Literature
79 results about "Vasodilating Agent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Any agent that causes widening of blood vessels as a result of smooth muscle relaxation.
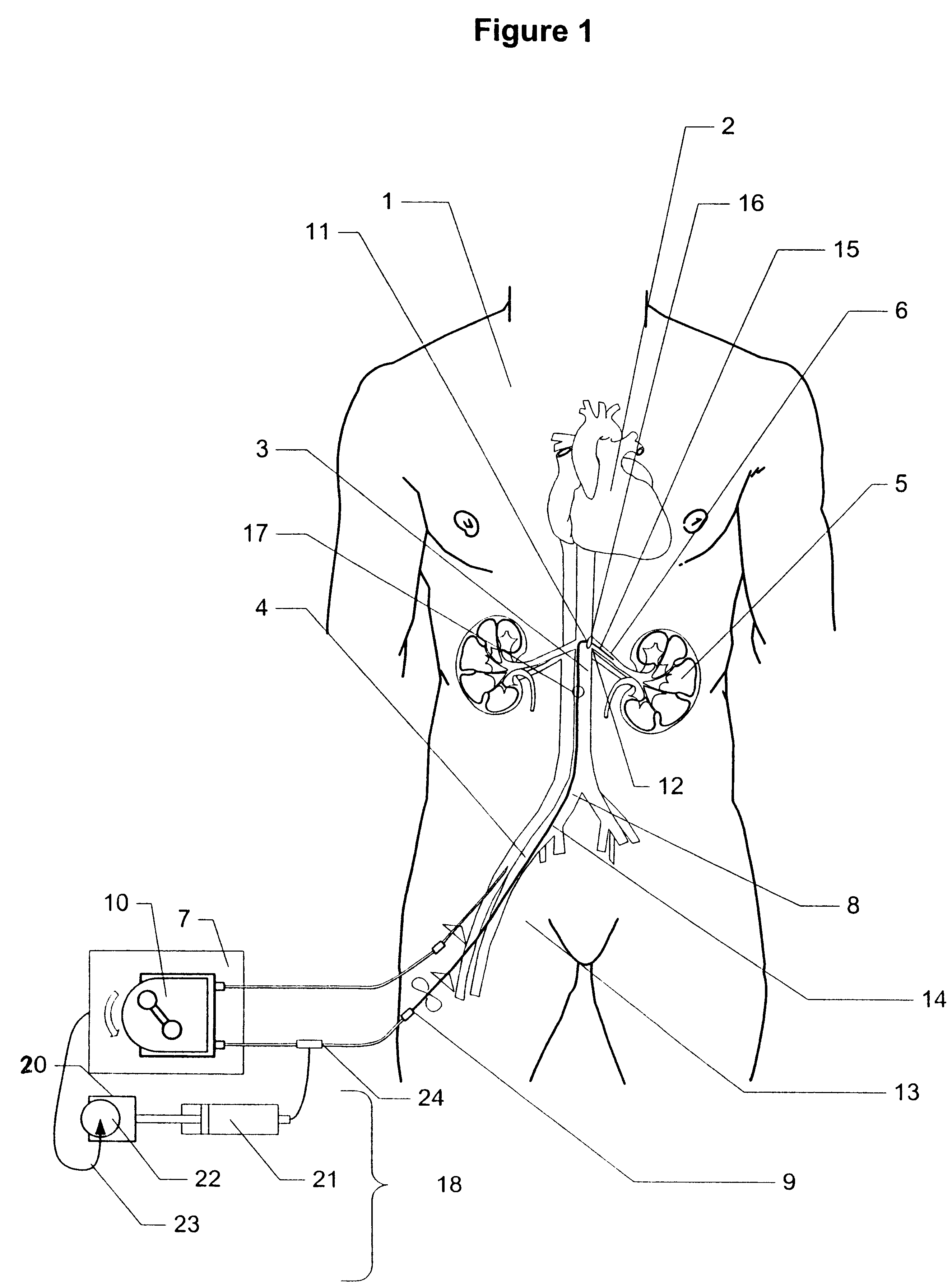
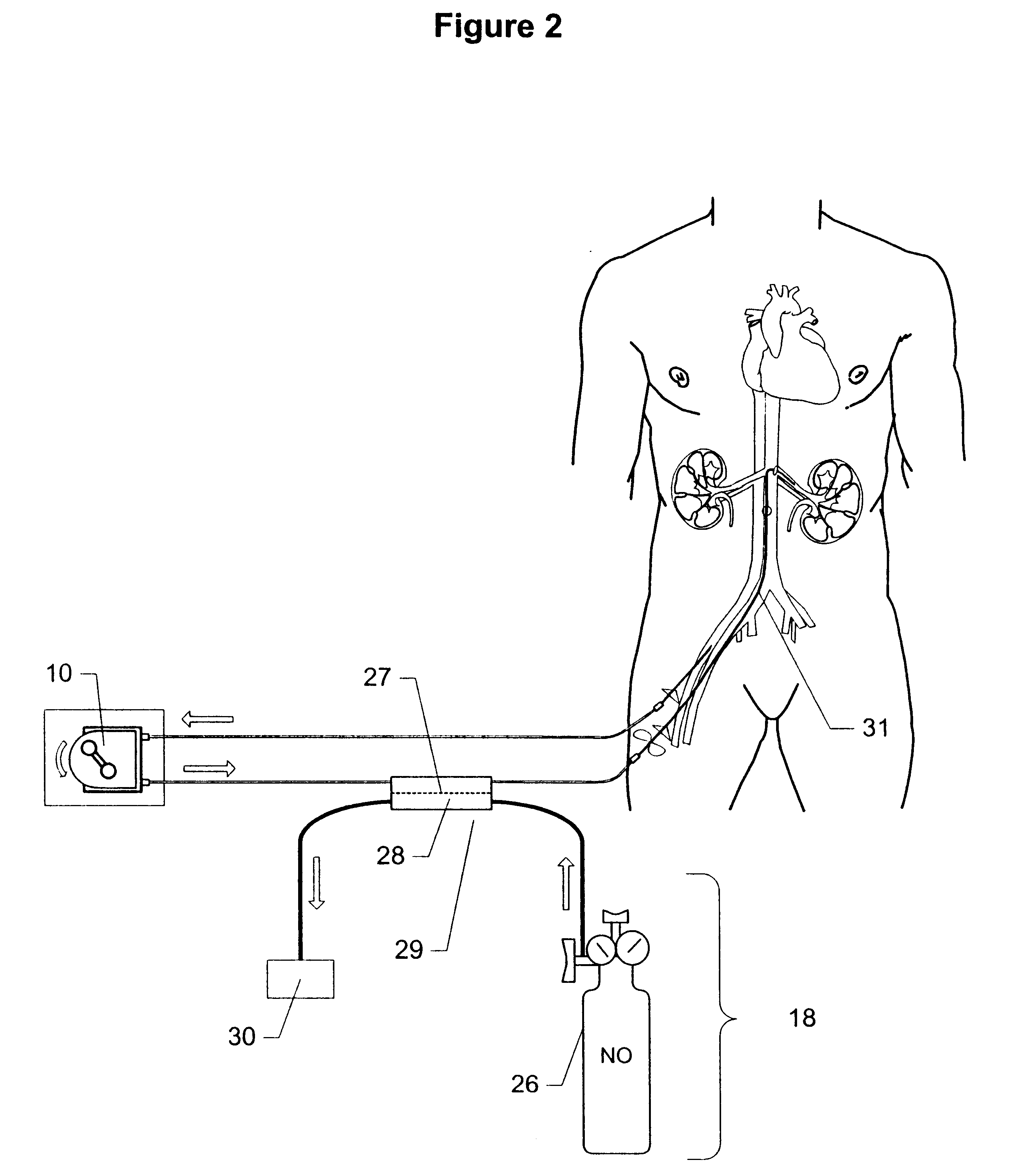
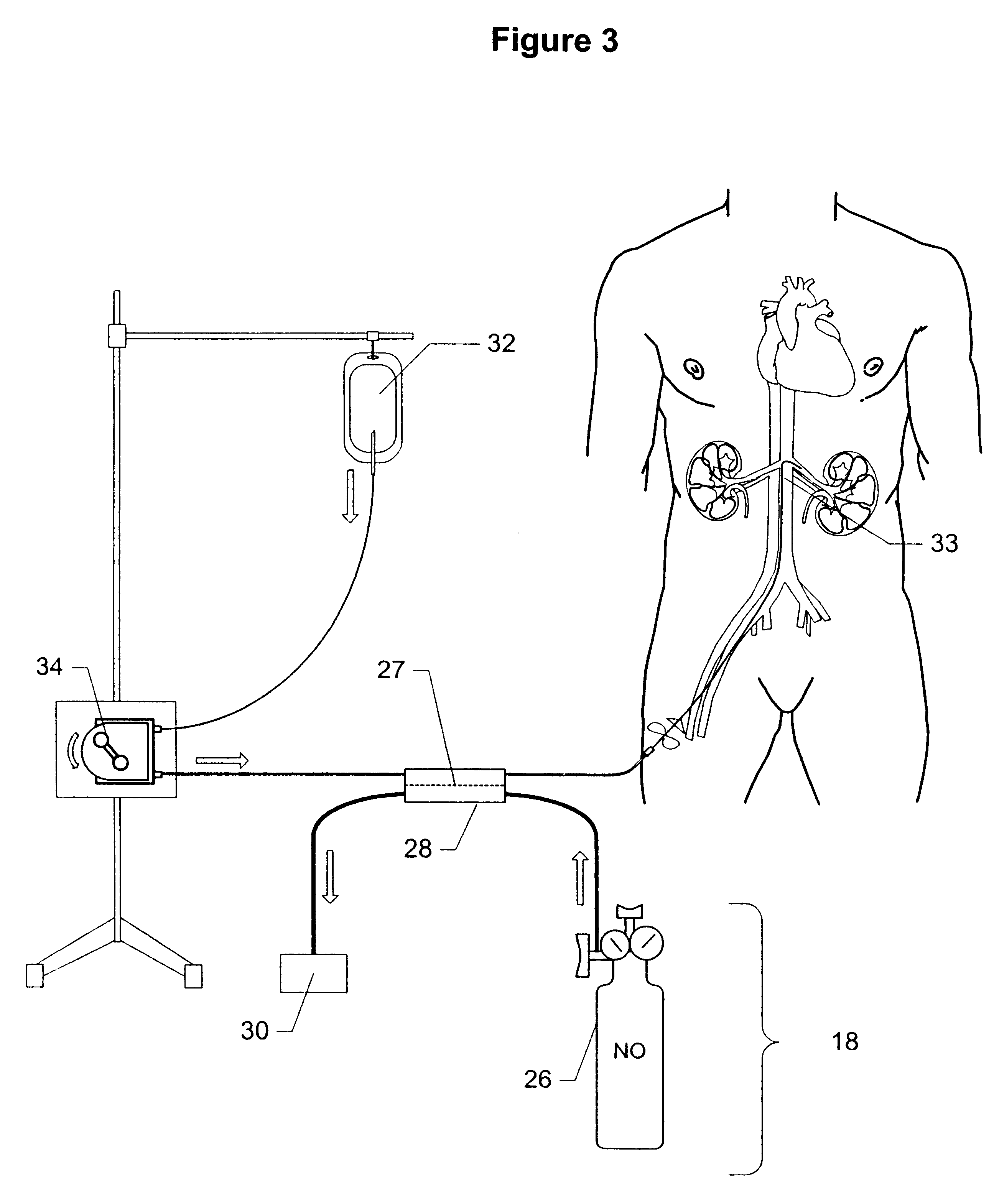
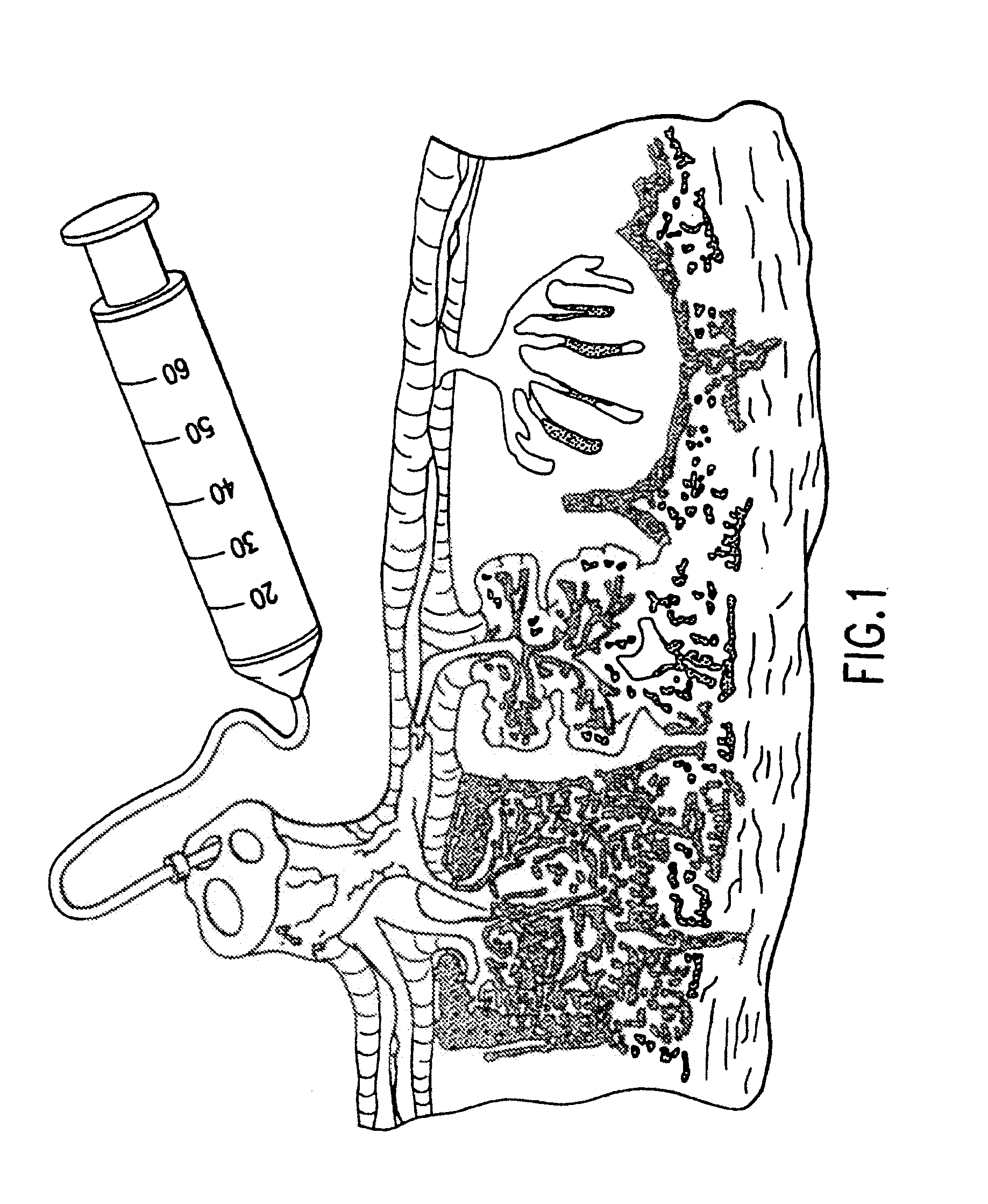
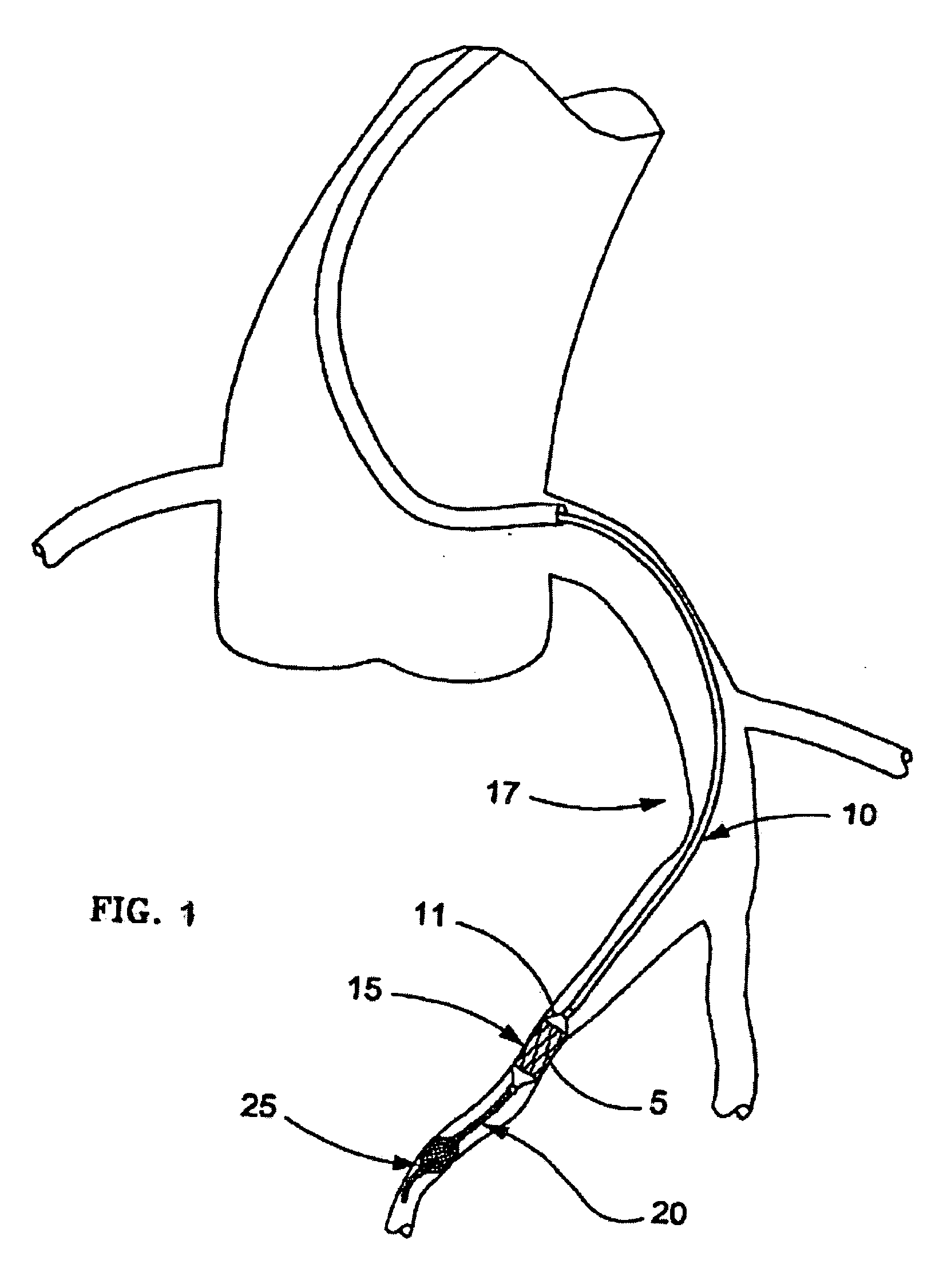
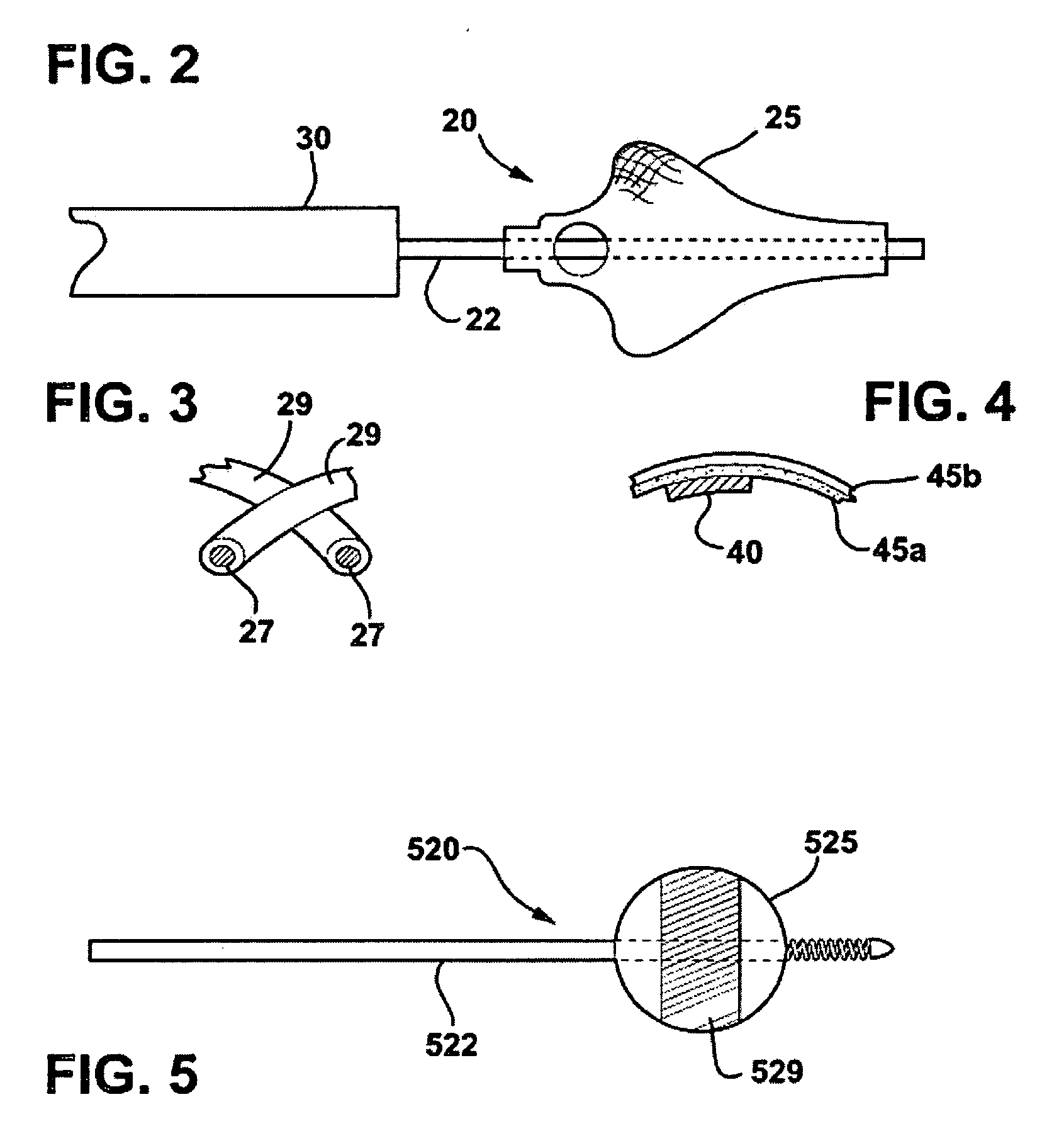
Method and apparatus for treatment of congestive heart failure by improving perfusion of the kidney by infusion of a vasodilator
InactiveUS6287608B1Improve the quality of lifeImprove survival rateBiocideInorganic active ingredientsRenin–angiotensin systemVascular dilatation
A method for treating congestive heart failure (CHF) has been developed that restores kidney renal functions by artificial vasodilation of at least one kidney. A vasodilator drug is locally delivered to the kidney via a kidney perfusion catheter. The drug can be mixed with the patient's blood, saline or other suitable solvent and the mixture directly applied to the kidney through the catheter. The restoration of kidney function assists the heart by removing excess fluid, urine and toxin from the patient, and by normalizing the patient's renin-angiotensin system and other neurohormonal substances. The method is applicable to treat chronic and acute CHF.
Owner:GAMBRO LUNDIA AB
Supplemented and unsupplemented tissue sealants, methods of their production and use
ActiveUS7189410B1Low antigenicityDecreasing thrombogenicityAntibacterial agentsOrganic active ingredientsTissue sealantVascular dilatation
This invention provides a fibrin sealant bandage, wherein said fibrin sealant may be supplemented with at least one composition selected from, for example, one or more regulatory compounds, antibody, antimicrobial compositions, analgesics, anticoagulants, antiproliferatives, anti-inflammatory compounds, cytokines, cytotoxins, drugs, growth factors, interferons, hormones, lipids, demineralized bone or bone morphogenetic proteins, cartilage inducing factors, oligonucleotides polymers, polysaccharides, polypeptides, protease inhibitors, vasoconstrictors or vasodilators, vitamins, minerals, stabilizers and the like. Also disclosed are methods of preparing and / or using the unsupplemented or supplemented fibrin sealant bandage.
Owner:AMERICAN NAT RED CROSS
Formulations and method for treating baldness
InactiveUS20060067905A1Lower Level RequirementsSlow, prevent, or even reverse hair lossCosmetic preparationsHair removalPersonal careApigenin
The present invention includes 1) a novel formulation for the treatment of hair loss comprising oleanolic acid (a 5α-reductase inhibitor), apigenin (a vasodilator), and biotinyl-GHK (a cell metabolism stimulant), 2) a novel additive for the treatment of hair loss comprising oleanolic acid, apigenin, biotinyl-GHK and a delivery agent, 3) a personal care, cosmetic, and / or dermopharmaceutical composition comprising oleanolic acid, apigenin, biotinyl-GHK, and at least one additional ingredient, and 4) a method for treating hair loss comprising the administration of oleanolic acid, apigenin, and biotinyl-GHK.
Owner:SEDERMA SA
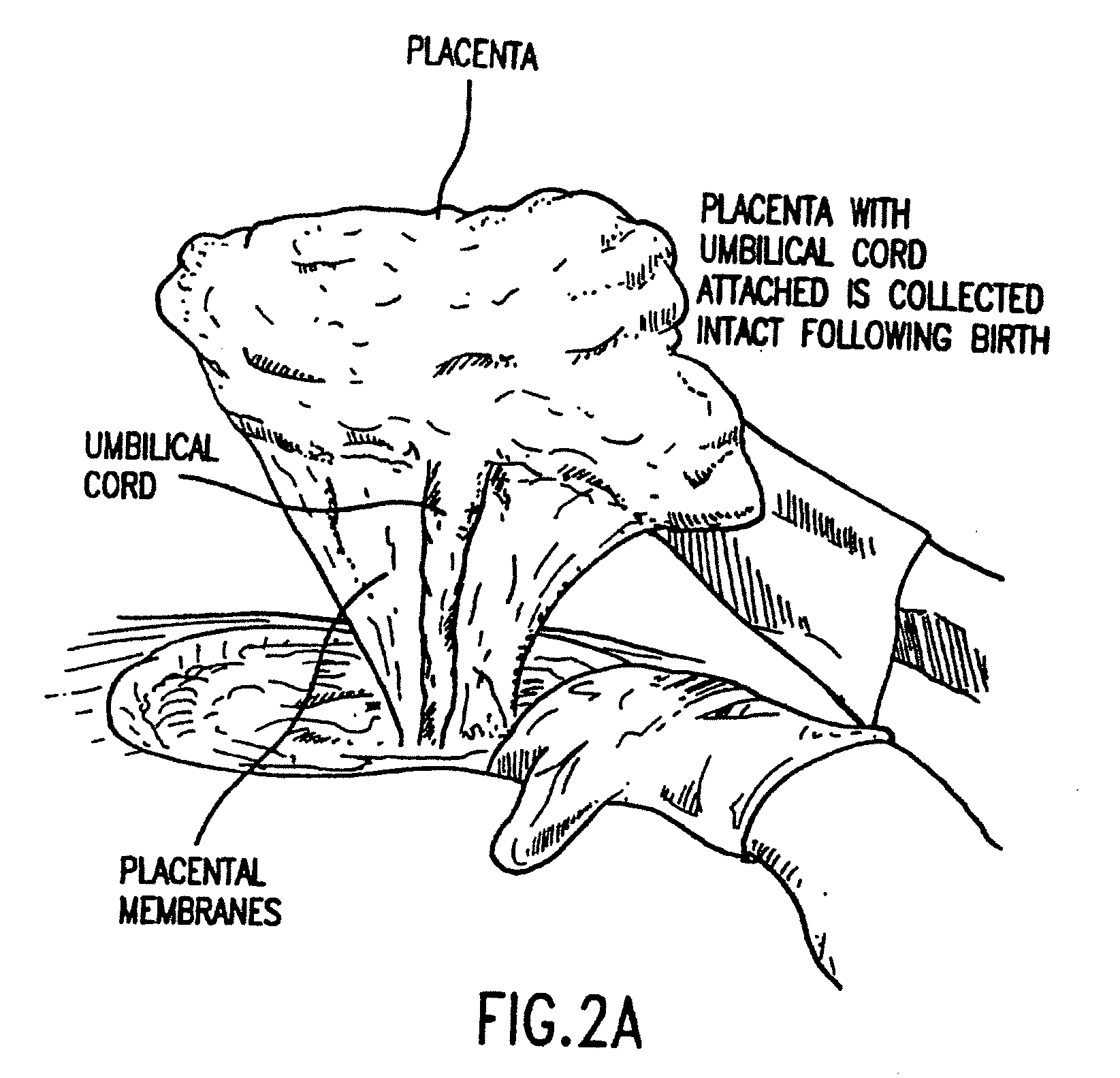
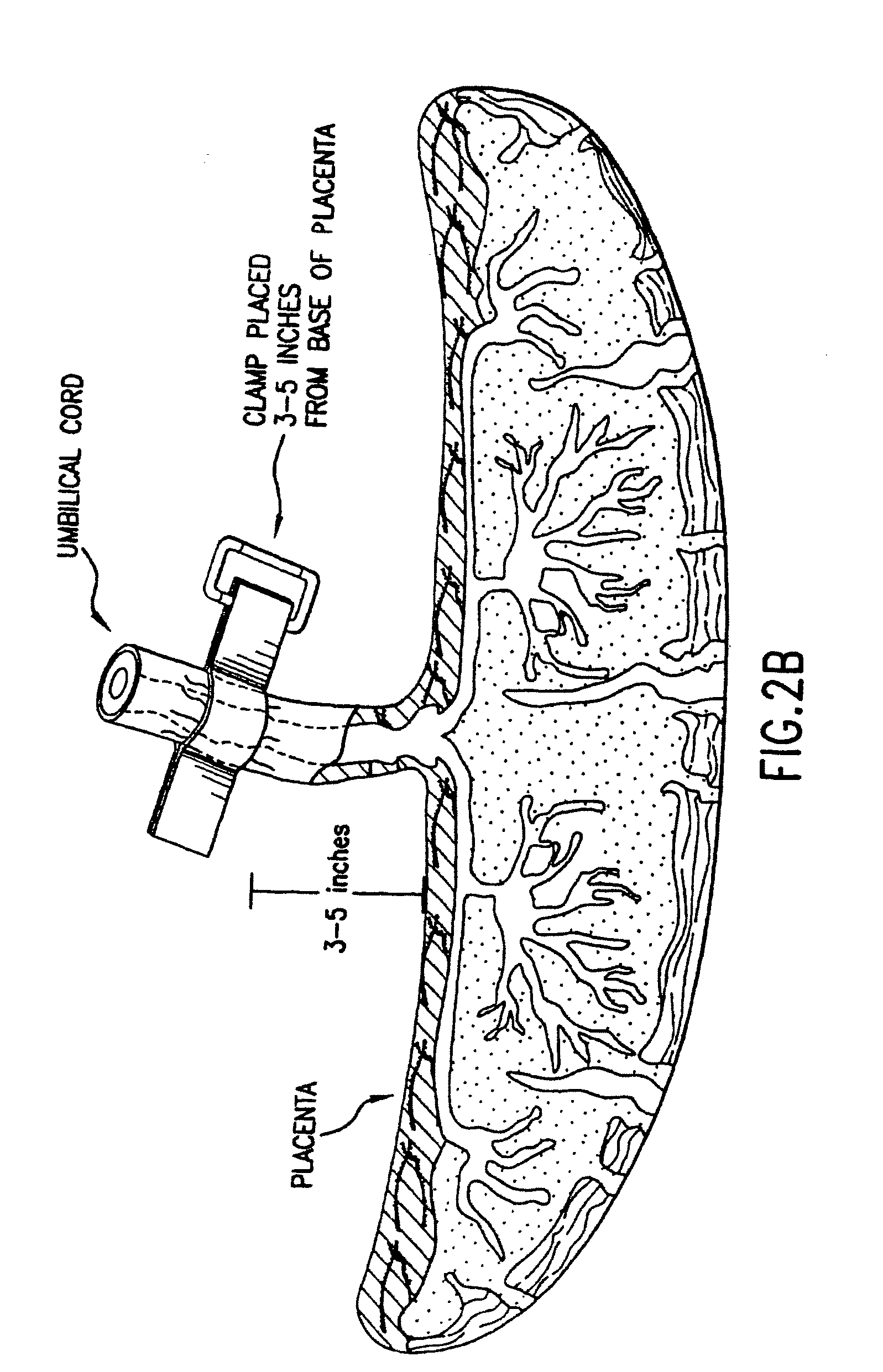
Composition for collecting and preserving placental stem cells and methods of using the composition
ActiveUS20100291679A1Cell culture active agentsNon-embryonic pluripotent stem cellsVascular dilatationBiology
The present invention provides improved compositions and methods for the collection of stem cells from an organ, e.g., placenta. The invention provides a stem cell collection composition comprising an apoptosis inhibitor and, optionally, an enzyme such as a protease or mucolytic enzyme, vasodilator, necrosis inhibitor, oxygen-carrying perfluorocarbon, or an organ preserving compound. The invention provides methods of using the stem cell collection composition to collect stem cells and to preserve populations of stem cells.
Owner:CELULARITY INC
Transdermal anesthetic and vasodilator composition and methods for topical administration
A composition for topical application comprising a therapeutically effective amount of a topical anesthetic, a safe and effective amount of a pharmaceutically acceptable topical vasodilator and a pharmaceutically acceptable carrier and a method of administering the composition to a mammal are disclosed.
Owner:SAMUELS PAUL J +1
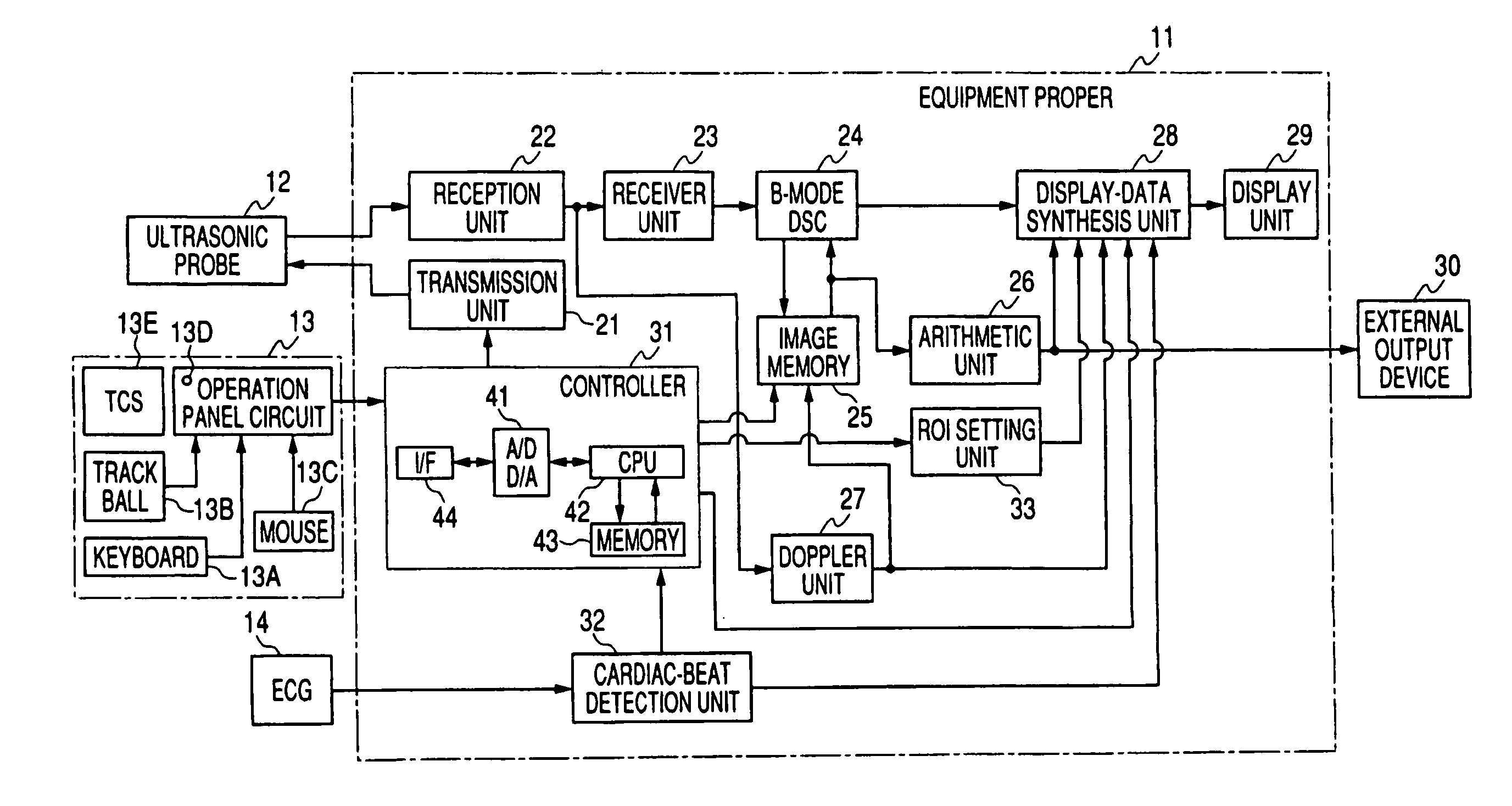
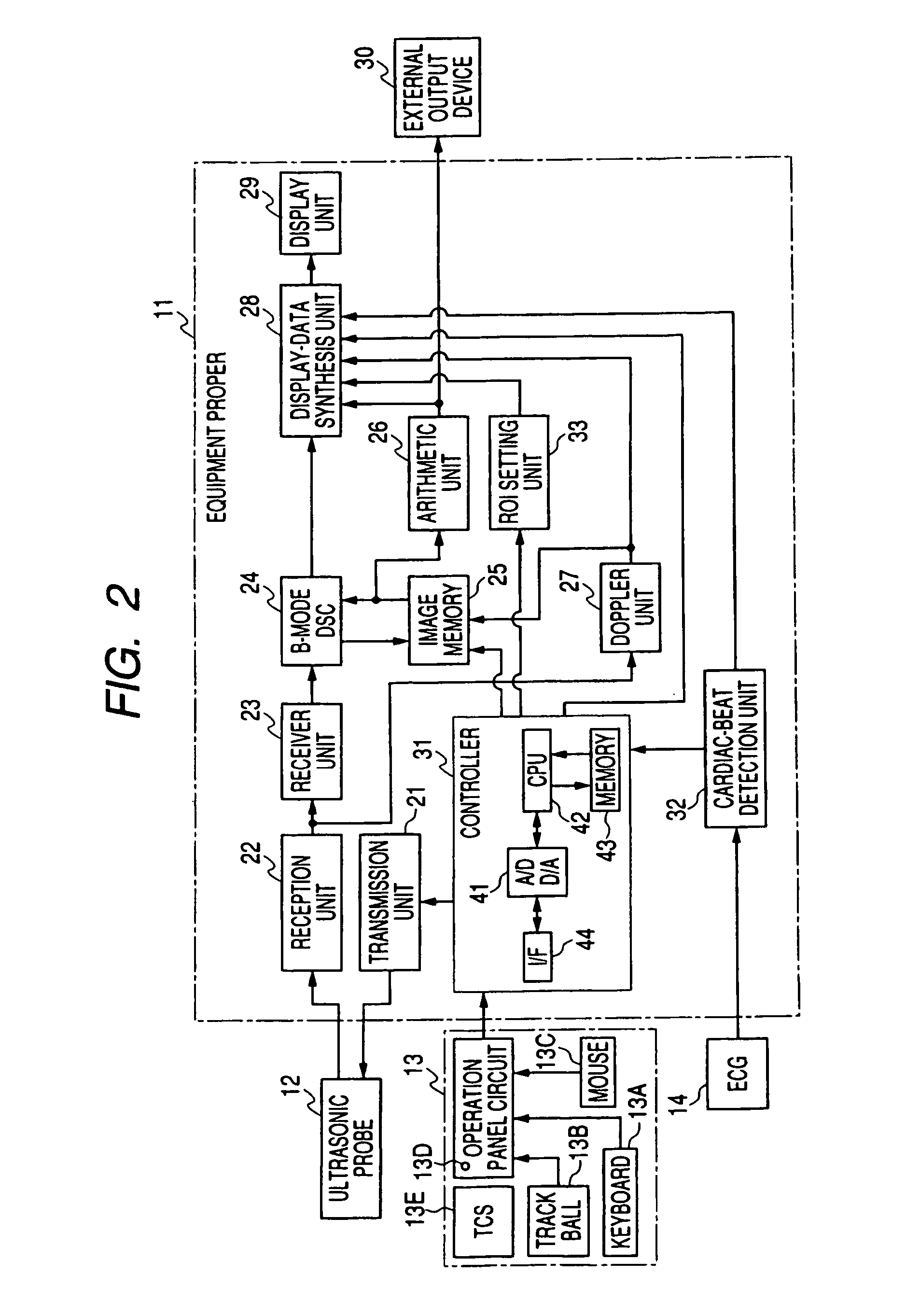
Ultrasonic diagnostic equipment and imaging processing apparatus
ActiveUS20050101863A1Accurate comparisonEasy and accurate settingBlood flow measurement devicesOrgan movement/changes detectionSonificationImaging processing
Each of an ultrasonic diagnostic apparatus and an image processing apparatus according to the present invention is characterized by comprising image acquisition means for acquiring image data by scanning a patient into whom an ultrasonic contrast medium has been injected, with ultrasonic beams, same-part correspondence means for bringing same parts into correspondence with each other, between a plurality of images concerning the same parts of the identical patient and acquired by the image acquisition means, arithmetic operation means for arithmetically operating image information items on changes of intensities between the plurality of images by using the intensities of those corresponding pixels of the plurality of images which have been brought into correspondence by the pixel correspondence means, and display means for displaying results operated by the arithmetic operation means. According to the construction, it is possible to provide the ultrasonic diagnostic equipment and the image processing apparatus which can compare the images before and after injection of a vasodilator drug, more accurately in contrast echo imaging.
Owner:TOSHIBA MEDICAL SYST CORP
Embolic protection apparatus with vasodilator coating
InactiveUS20060282114A1Reduce and prevent incidencePrevent embolismDilatorsExcision instrumentsVascular dilatationEmbolic Protection Devices
An apparatus for temporary prevention of embolization in a human blood vessel. The apparatus comprises a body that is transformable between a radially collapsed configuration and a radially expanded configuration sized and shaped for sealing against an inner wall of the vessel to at least partially obstruct fluid flowing there through, the body having a vasodilator coating.
Owner:MEDTRONIC VASCULAR INC
Supplemented and unsupplemented tissue sealants, methods of their production and use
InactiveUSRE39192E1Increased longevityImprove stabilityAntibacterial agentsPowder deliveryTissue sealantVascular dilatation
This invention provides supplemented tissue sealants, methods for their production and use thereof. Disclosed are tissue sealants supplemented with at least one cytotoxin or cell proliferation inhibiting composition. The composition may be further supplemented with, for example, one or more antibodies, analgesics, anticoagulants, anti-inflammatory compounds, antimicrobial compositions, cytokines, drugs, growth factors, interferons, hormones, lipids, deminearlized bone or bone morphogenetic proteins, cartilage inducing factors, oligonucleotides polymers, polysaccharides, polypeptides, protease inhibitors, vasoconstrictors or vasodilators, vitamins, minerals, stabilizers and the like.
Owner:AMERICAN NAT RED CROSS
Supplemented and unsupplemented tissue sealants, methods of their production and use
InactiveUSRE39298E1Decreasing thrombogenicityLow antigenicityOrganic active ingredientsPowder deliveryTissue sealantVascular dilatation
This invention provides methods for the localized delivery of supplemented tissue sealants, wherein the supplemented tissue sealants comprise at least one composition which is selected from one or more antibodies, analgesics, anticoagulants, anti-inflammatory compounds, antimicrobial compositions, antiproliferatives, cytokines, cytotoxins, drugs, growth factors, interferons, hormones, lipids, demineralized bone or bone morphogenetic proteins, cartilage inducing factors, oligonucleotides polymers, polysaccharides, polypeptides, protease inhibitors, vasoconstrictors or vasodilators, vitamins, minerals, stabilizers and the like. Further provided are methods of using the site-specific supplemented tissue sealants, including preparation of a biomaterial.
Owner:AMERICAN NAT RED CROSS
Cosmetic herbal compositions
InactiveUS20070196318A1Reduce oxidative stressReduce inflammationCosmetic preparationsBiocideActive agentVascular dilatation
Herbal cosmetic skin care compositions formulated to combat conditions associated with oxidative stress are provided. The compositions contain effective amounts of one of several active agents, including an herbal agent derived from one or more of the plant species, as well as cosmetically acceptable carriers. Other agents that may be included are proteins, peptides, anti-inflammatory agents, and / or vasodilators. These cosmetic compositions find use in improving the appearance of aged or damaged skin.
Owner:JAN MARINI SKIN RES
Medicinal composition for prevention of or treatment for cerebrovascular disorder and cardiopathy
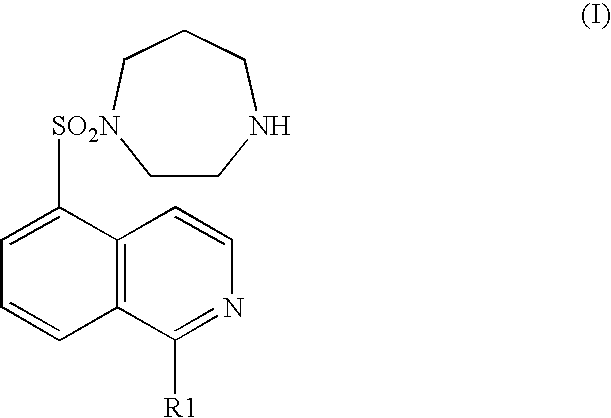
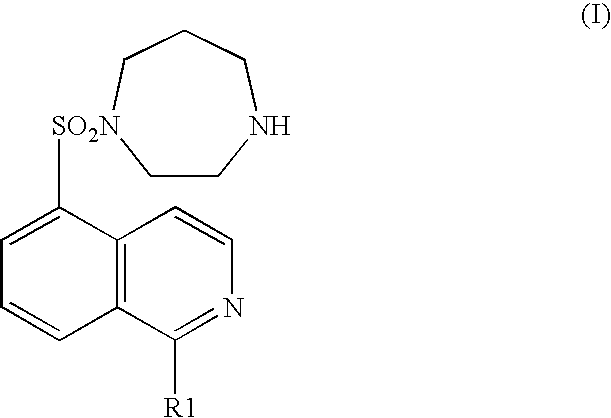
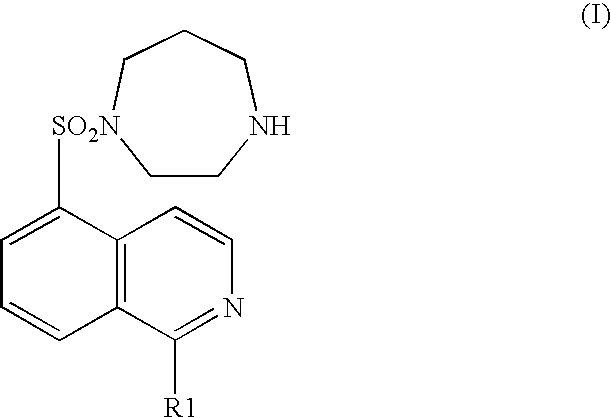
A pharmaceutical composition comprising at least one of components (a) and at least one of components (b) shown in below: (a) a compound represented by the general formula (I) (wherein R1 represents a hydrogen atom or a hydroxyl group) or an acid addition salt or hydrate thereof; and (b) an ameliorant of cerebral circulation, a vasodilator, a cerebral protecting drug, an brain metabolic stimulants, an anticoagulant, an antiplatelet drug, a thrombolytic drug, an amelirant of psychiatric symptom, a antihypertensive drug, an antianginal drug, a diuretic, a cardiotonic, an antiarrhythmic drug, an antihyperlipidemic drug, an immunosuppressant, or a pharmaceutically acceptable salt (except the components shown in (a)). It is useful as a preventive or remedy for cerebrovascular disorders and cardiac diseases.
Owner:ASAHI KASEI PHARMA
Transdermal drug delivery formulations with optimal amounts of vasodilators therein
InactiveUS20060013866A1Maximize efficiencyEasy to transportSalicyclic acid active ingredientsBiocideVascular dilatationDelivery vehicle
Topical drug delivery formulations with optical amounts of vasodilator. Vasodilator chemicals applied topically dilate the blood vessels in the skin tissue, which have been shown to facilitate or inhibit systemic or skin tissue deposition of drug substances. The level of stimulation and / or inhibition has been found to be dependent on the concentration and the identity of the specific vasodilator chemical(s) used as well as the drug molecule(s) to be delivered. This work teaches the need to consider specific formulation requirements when dealing with vasodilator chemicals for the creation of successful delivery vehicles in the transdermal drug delivery system. These requirements for very low concentrations of vasodilators were an unexpected and a surprise finding, in contrast to the concentrations of the vasodilators typically used to elicit an increase in skin blood flow.
Owner:BIOCHEMICS
Compositions for protection against superficial vasodilator flush syndrome, and methods of use
ActiveUS20070141187A1Promote absorptionBiocideCosmetic preparationsSulfate proteoglycanVascular dilatation
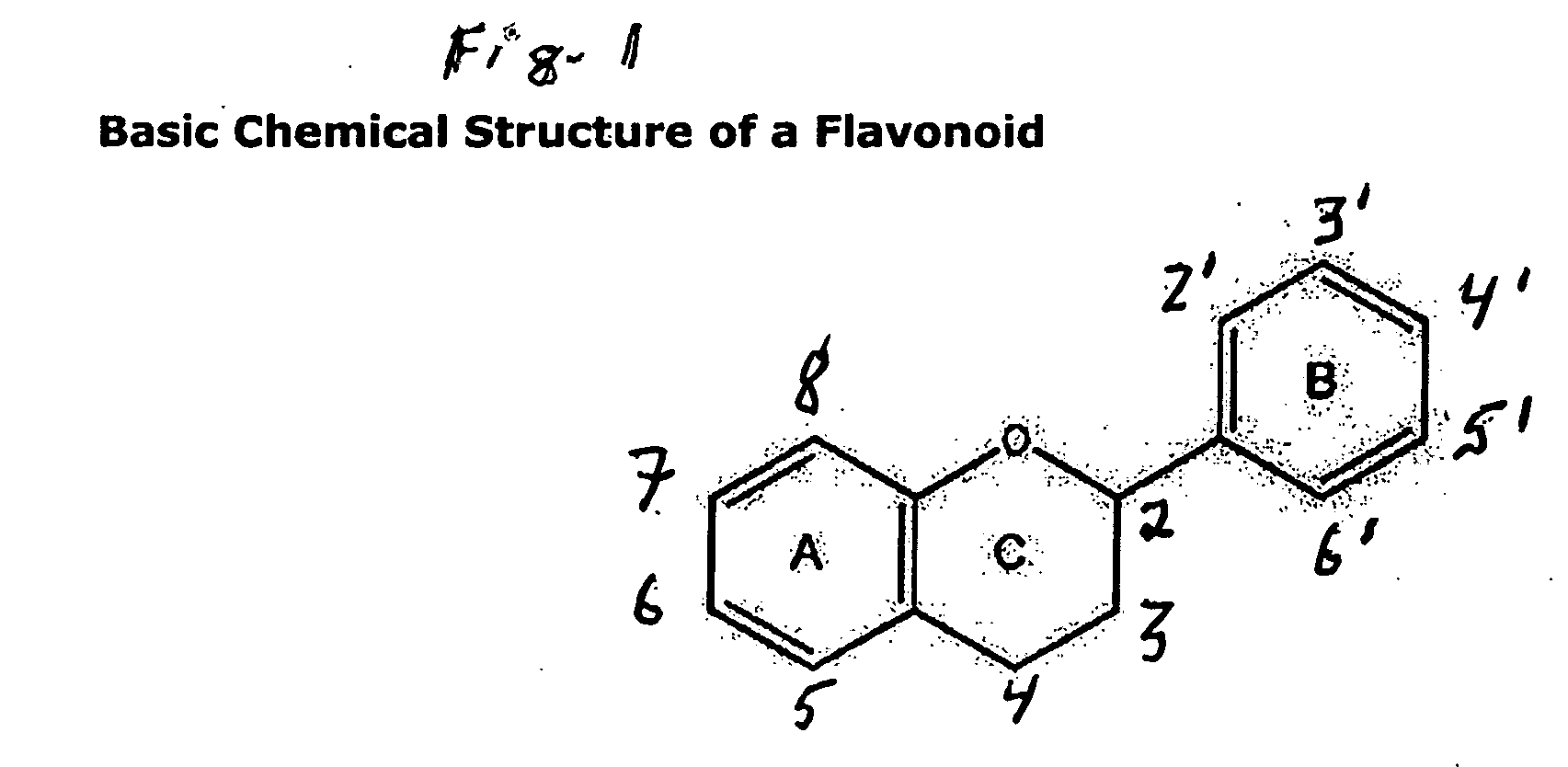
Compositions for protection against SVFS syndromes are composed of a flavonoid compound of the basic structures 2-phenyl-4H-1-benzopyran or 2-phenyl-4-keto-1-benzopyran or glycosides thereof, an inventive olive kernel extract and a non-bovine sulfated proteoglycan, and, optionally, one or more of bitter willow extract, D-glucosamine sulfate and serotonin inhibitor.
Owner:THETA BIOMEDICAL CONSULTING & DEVMENT
Compositions methods for improving cardio vascular function
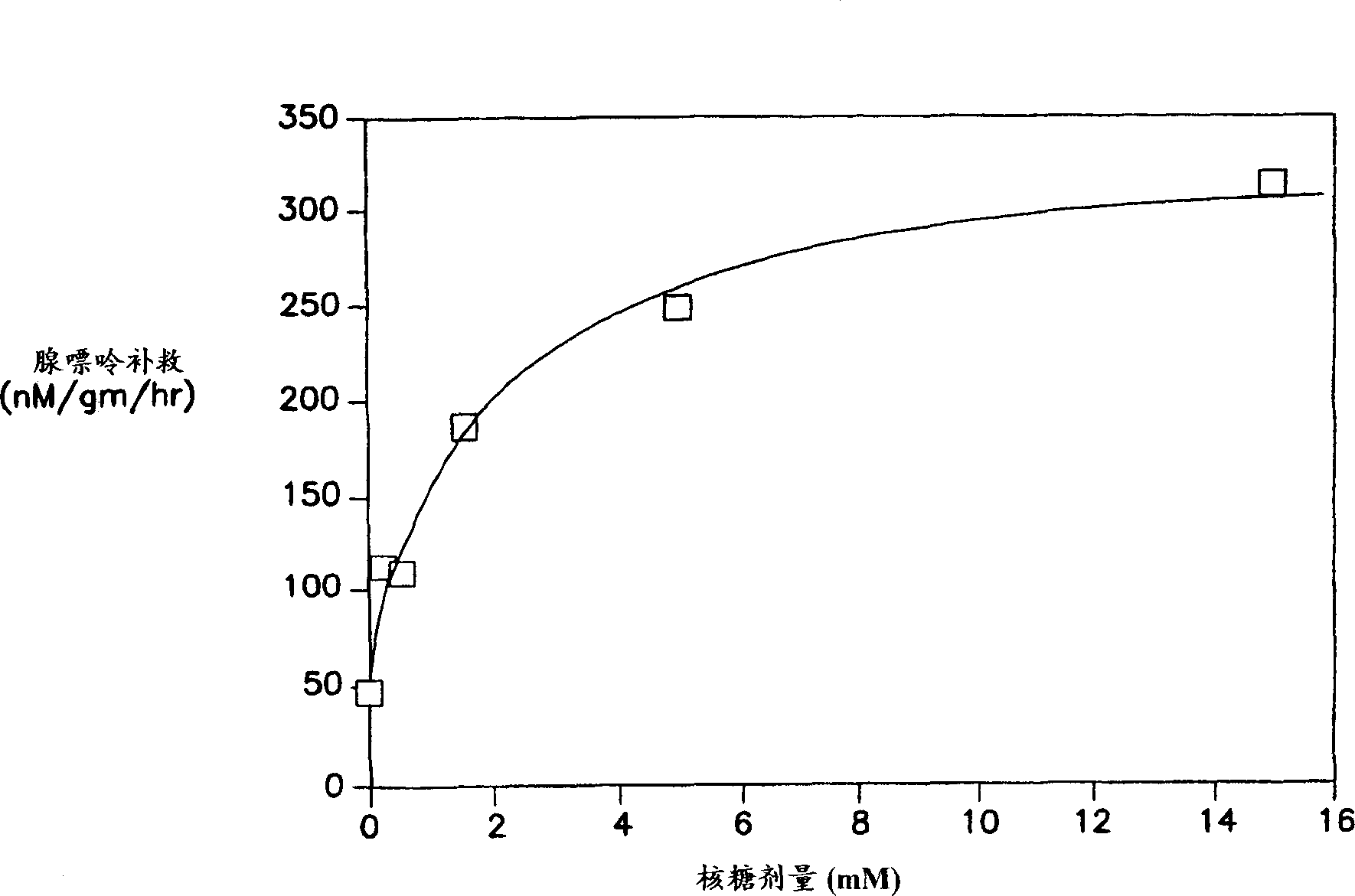
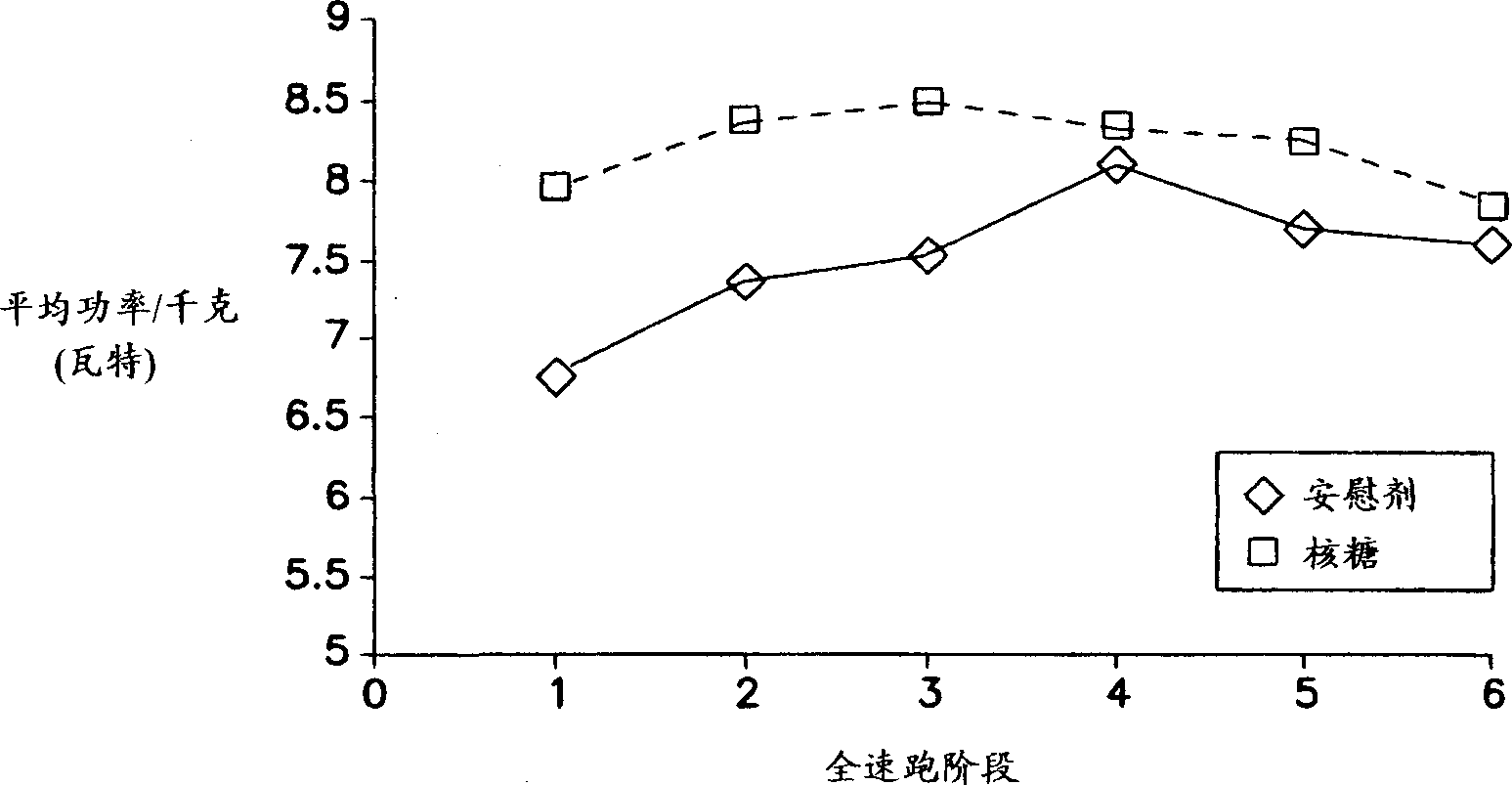
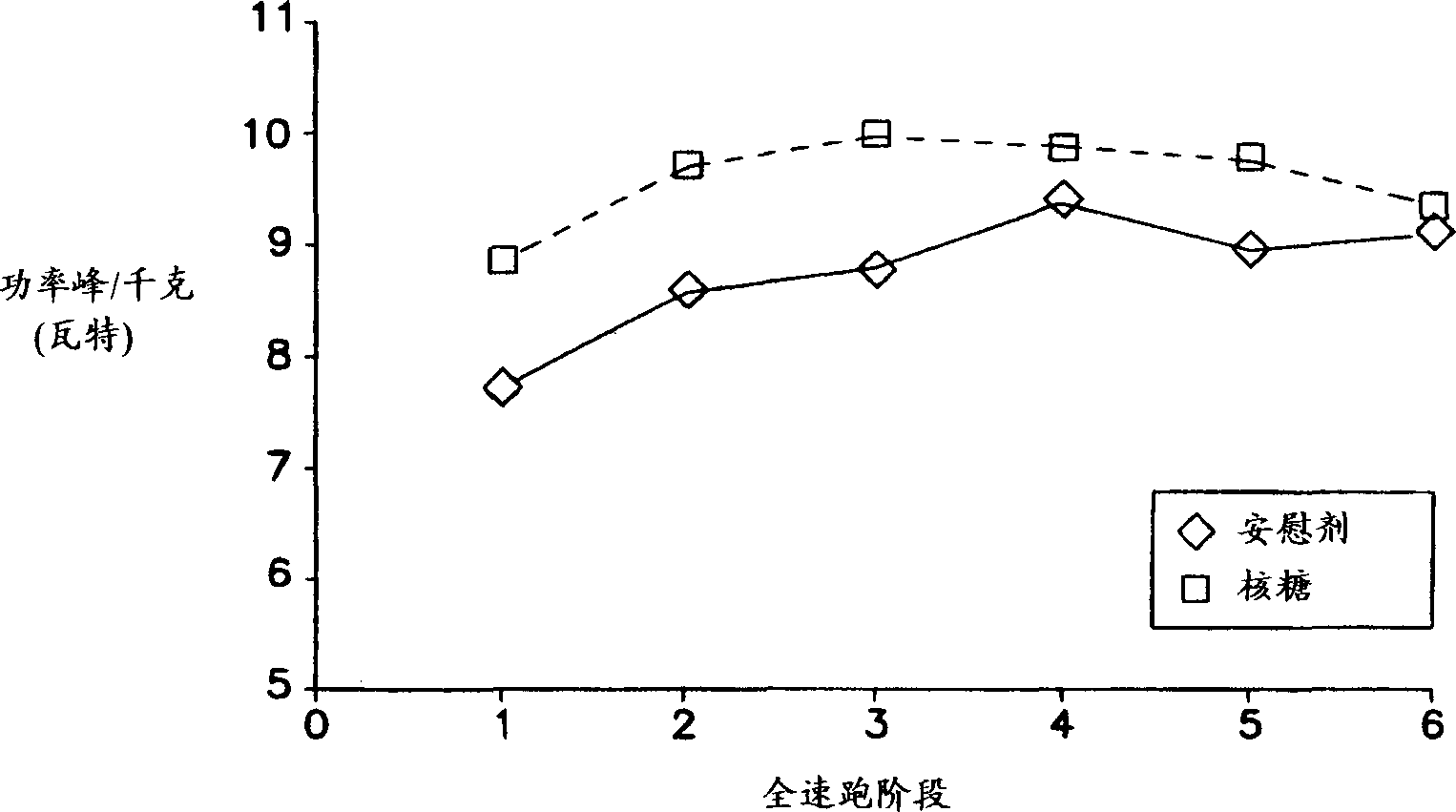
The present invention relates to compositions for supplementing the diet of subjects suffering from cardiovascular or peripheral vascular disease or those at risk for such conditions. Ribose is given alone or in combination with one or a combination of vasodilators, nutrients and vitamins. Preferred vitamins include Vitamins C, B6, B12 and folic acid. Preferred nutrients include glutamine and glucose.
Owner:BIOENERGY INC
Inhalable formulations for treating pulmonary hypertension and methods of using same
The present invention is directed to an inhalable formulation for the treatment of pulmonary hypertension in a mammal (e.g., humans), wherein the formulation comprises at least one hypertension reducing agent, including but not limited to an angiotensin converting enzyme inhibitor, angiotensin receptor blocker, beta-blocker, calcium-channel blocker or vasodilator, or any combination thereof. The formulations of the present invention may be a solution or suspension, and preferably are suitable for administration via nebulization. The present invention is also directed to a method and kit for treating a mammal suffering from pulmonary hypertension.
Owner:MYLAN SPECIALTY
Derivatives of isosorbide mononitrate and its use as vasodilating agents with reduced tolerance
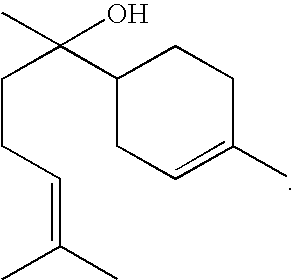
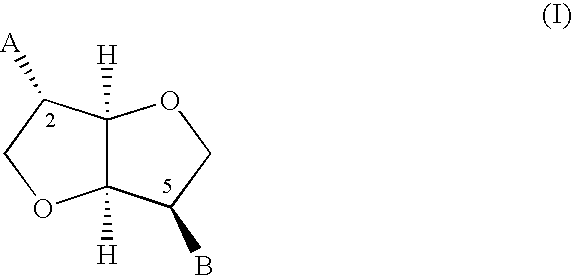
InactiveUS6858632B2Potent vasodilating effectSmall and null tolerance effectBiocideOrganic chemistryArylTolerability
Novel derivatives of isosorbide mononitrate and its pharmaceutically acceptable salts, which have vasodilating activity with a reduced effect of tolerance, of the general formula (I) in which A and B independently represent any of the groups—ONO2 and —Z—CO—R, wherein Z is an oxygen atom or sulphur atom and R is an alkyl C1-C4 group, an aryl group or an aralkyl group, eventually substituted, or the group in which R1 is hydrogen, or an alkyl C1-C4 group, an aryl group or an aralkyl group, eventually substituted, with the proviso that one of A or B is always —ONO2, but never both of them at the same time, when Z is an sulphur atom R is an alkyl C1-C4 group, an aryl group or an aralkyl group, eventually substituted, and when Z is an oxygen atom R is the group
Owner:LACER SA
Compositions for protection against superficial vasodilator flush syndrome, and methods of use
InactiveUS20120046237A1Good effectGood water solubilityBiocideOrganic chemistryVascular dilatationPhospholipid
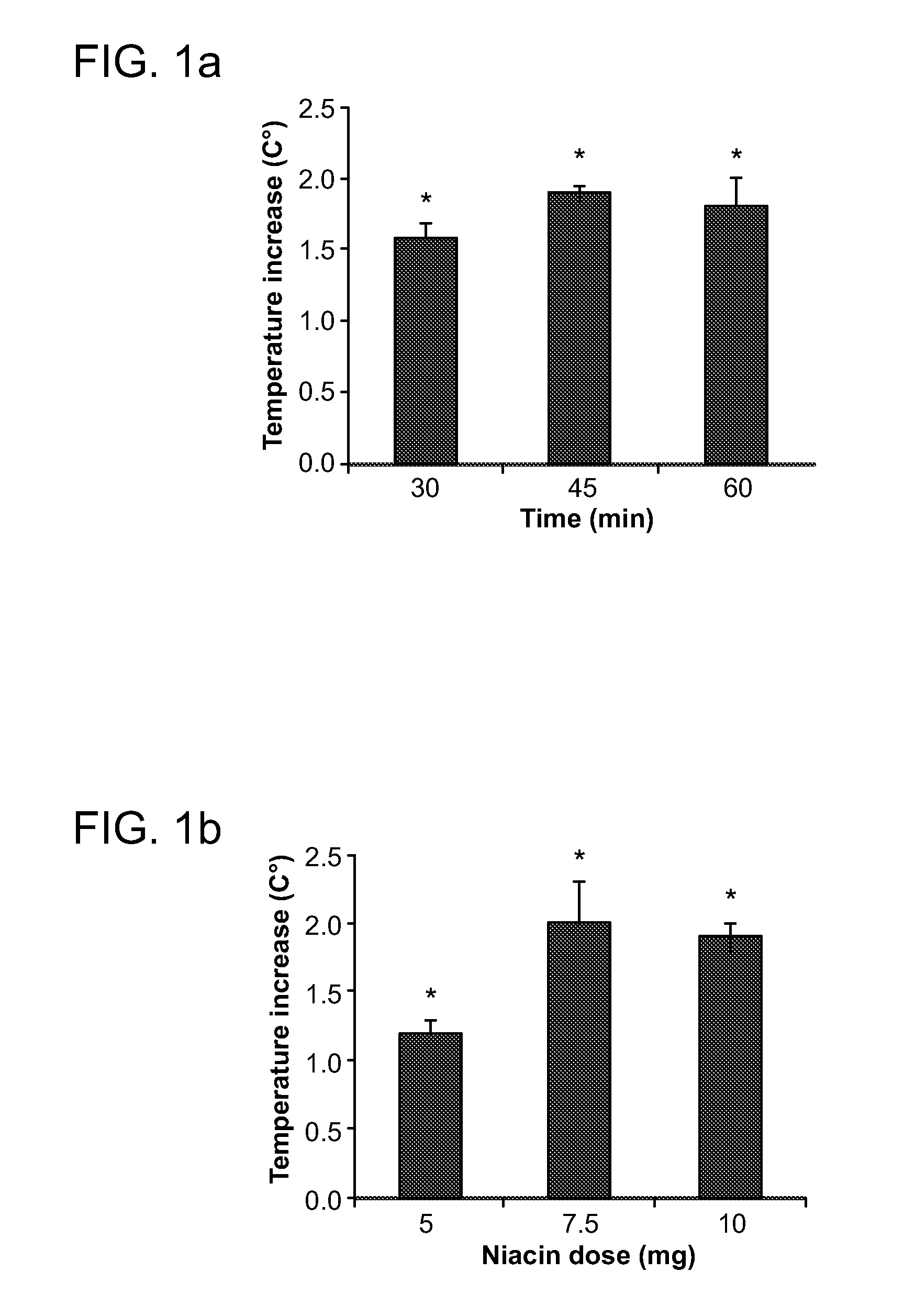
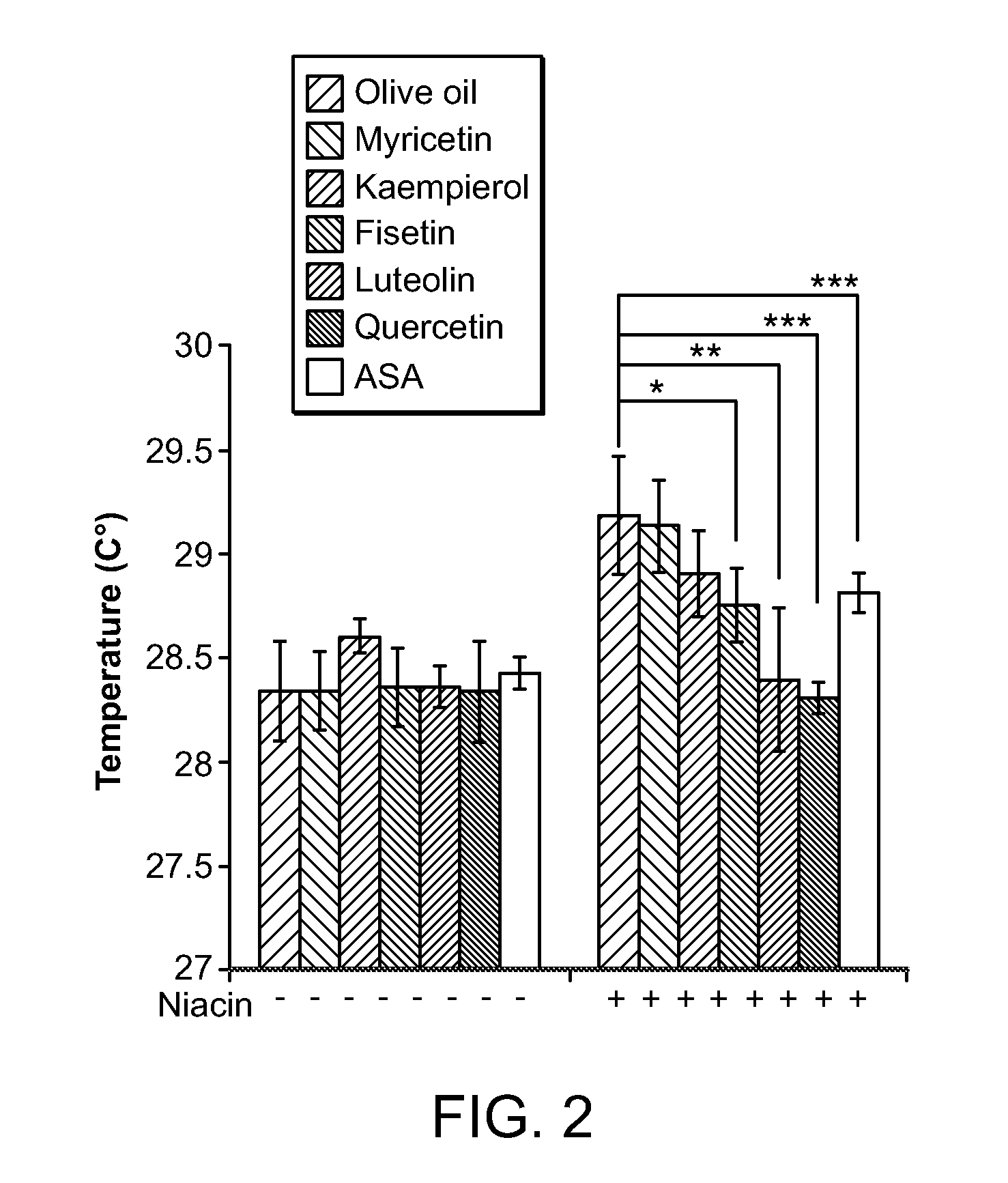
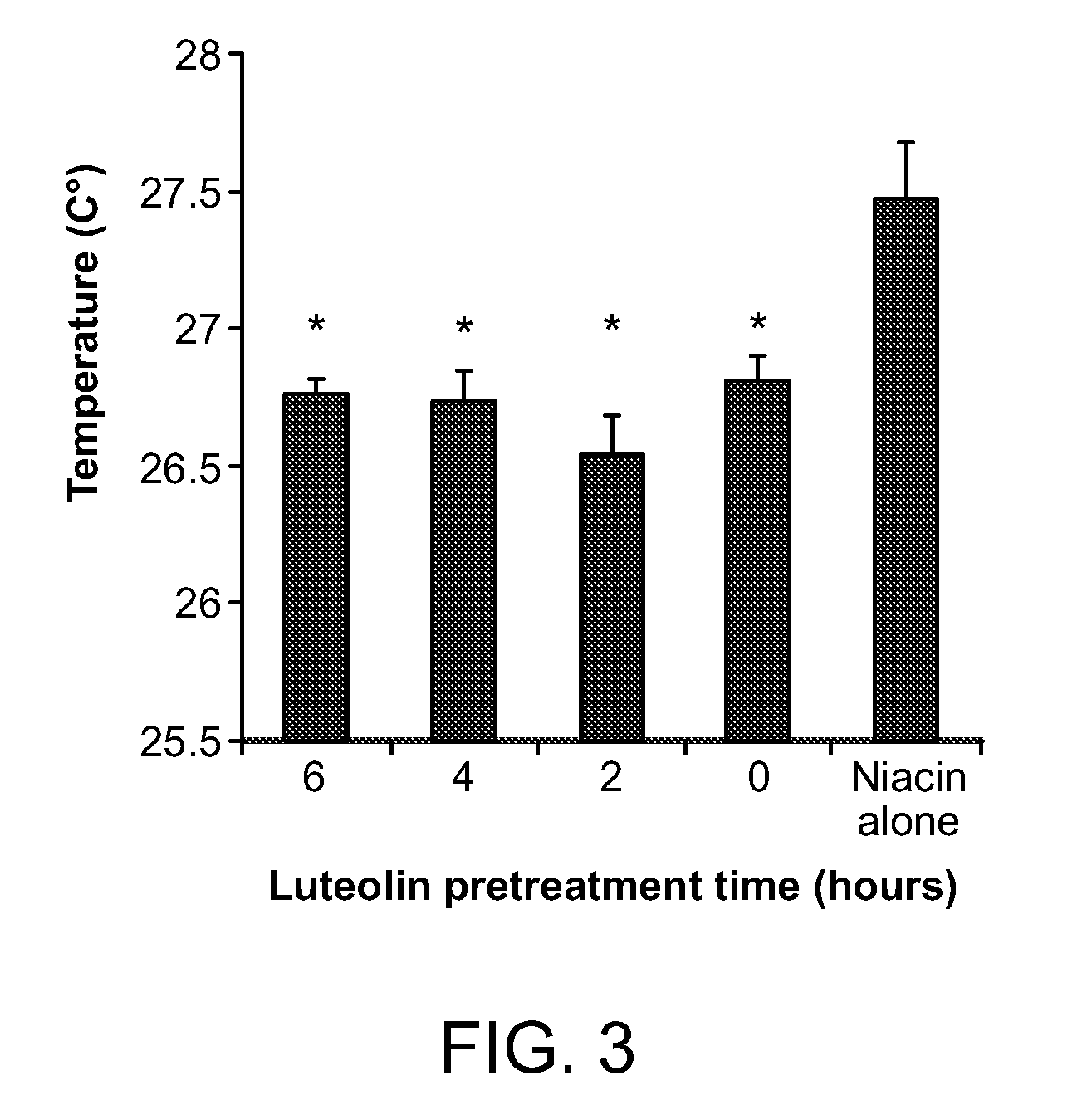
Compositions for protection against SVFS induced by niacin, a carcinoid, mesenteric traction, serotonin, post-menopause, alcohol, monosodium glutamate, mastocytosis, atopic dermatitis, food-allergy or food intolerance, and mast cell activation syndrome, or against individual symptoms of SVFS, superficial vasodilation, feeling of warmth, itching (pruritus) and hives, comprising a flavonoid compound of the structure 2-phenyl-4H-1-benzopyran or 2-phenyl-4-keto-1-benzopyran or glycosides thereof, or chalconoid compounds, with appropriate substitutions of their hydroxyl groups to render them water soluble or in combination with a pshospholipid or cyclodextrin to render them to have higher oral absorption, administered alone or together with an anti-superficial vasodilation dose of one or more of, olive kernel oil, a serotonin inhibitor, a prostaglandin inhibitor, willow bark extract. A composition for treating cardiovascular disease with niacin, but without eliciting the SVFS effects of niacin, has also been invented.
Owner:THETA BIOMEDICAL CONSULTING & DEVMENT
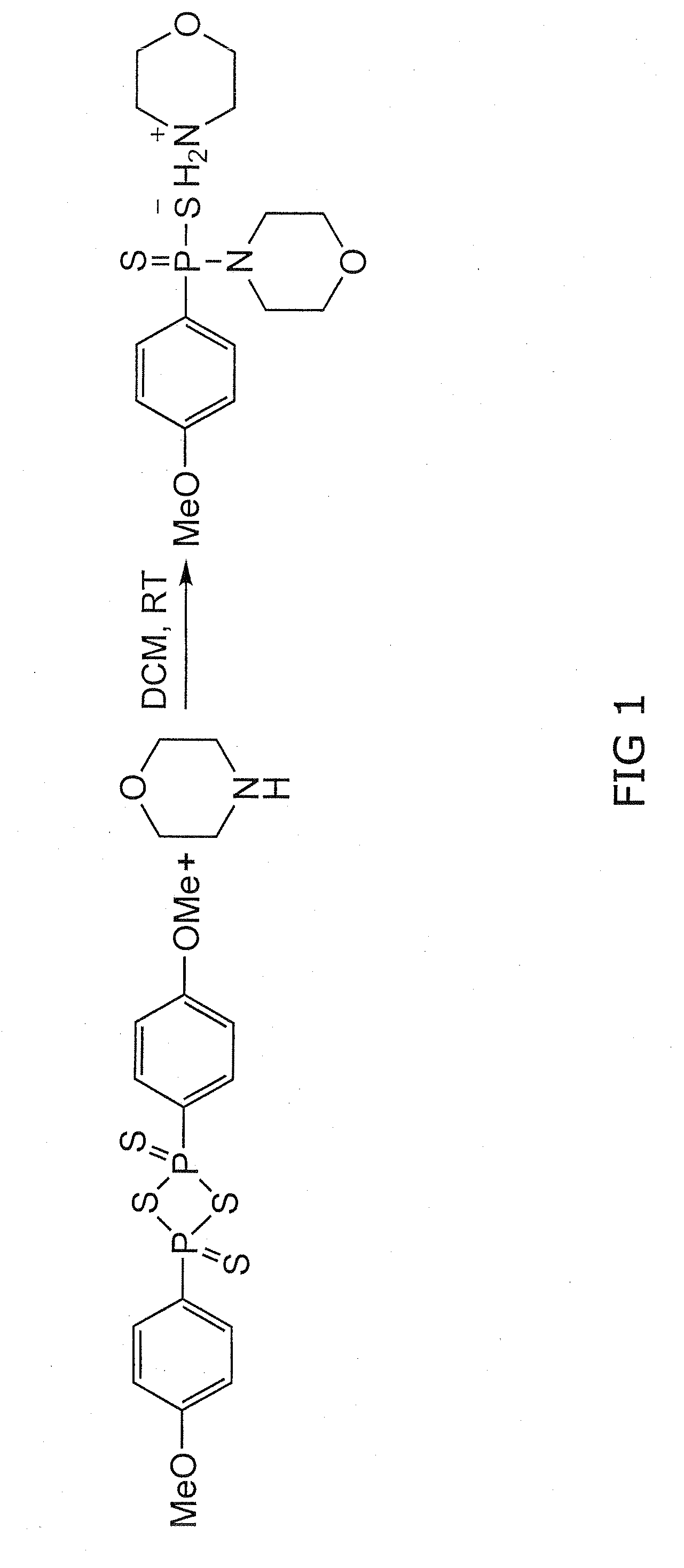
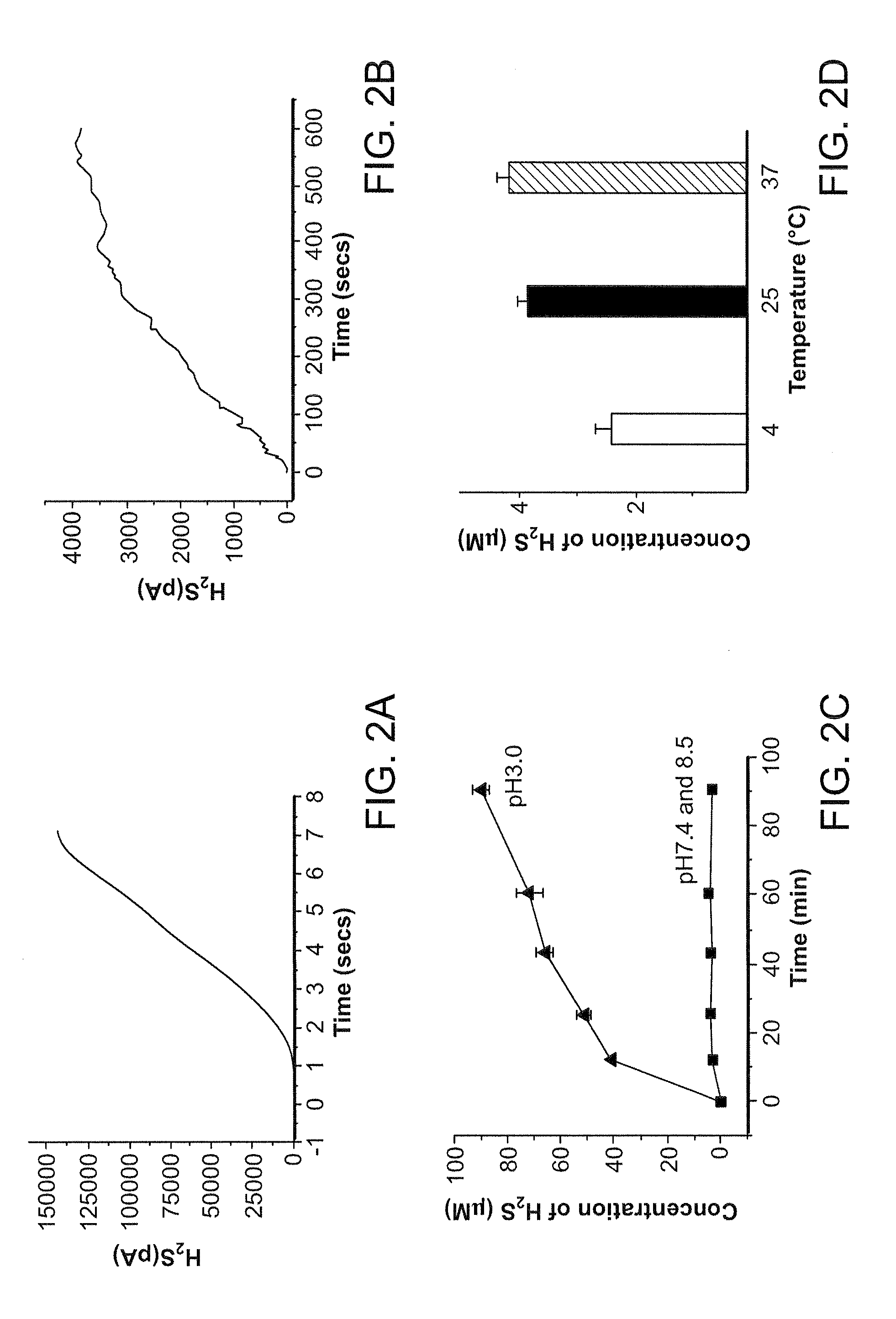
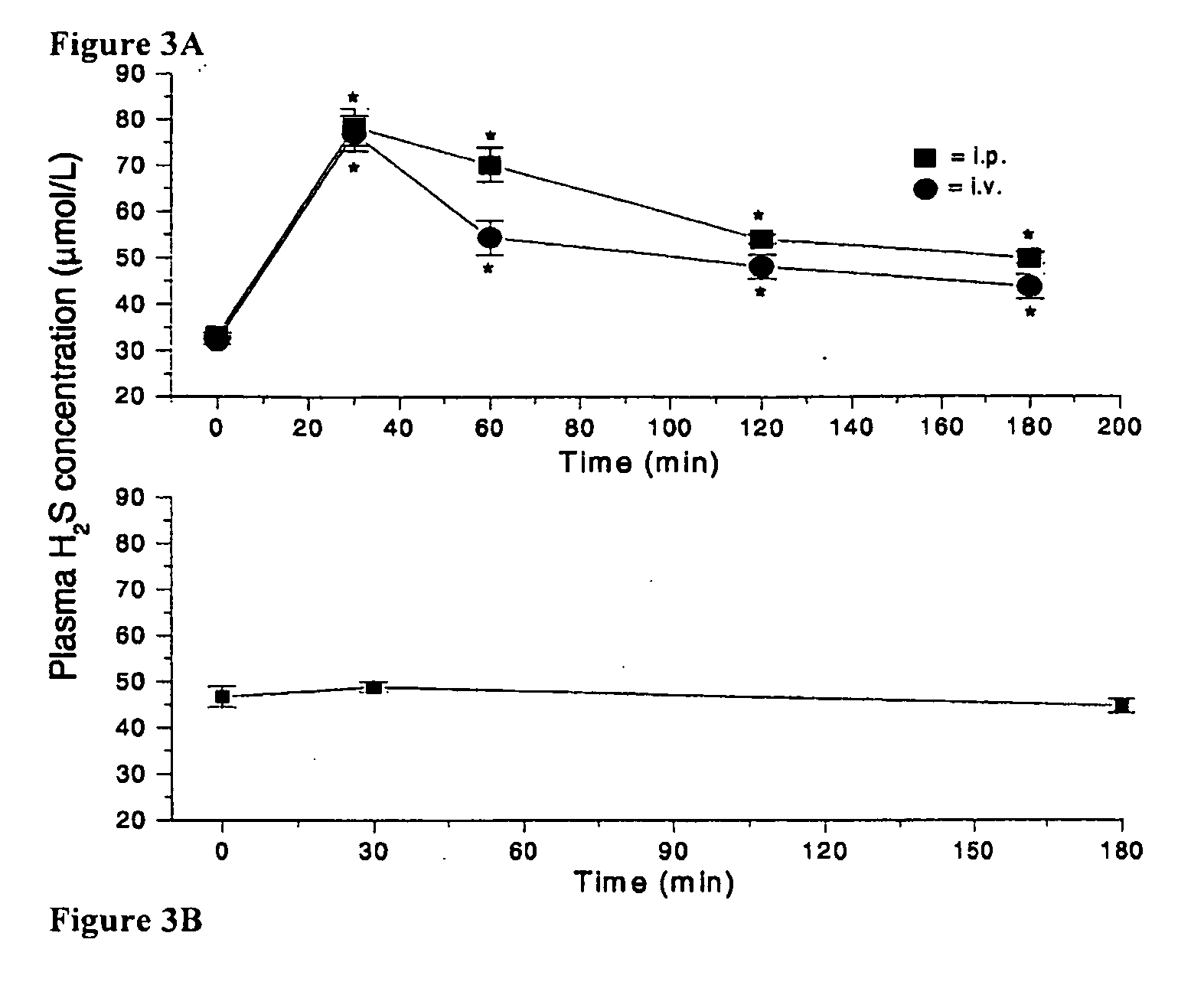
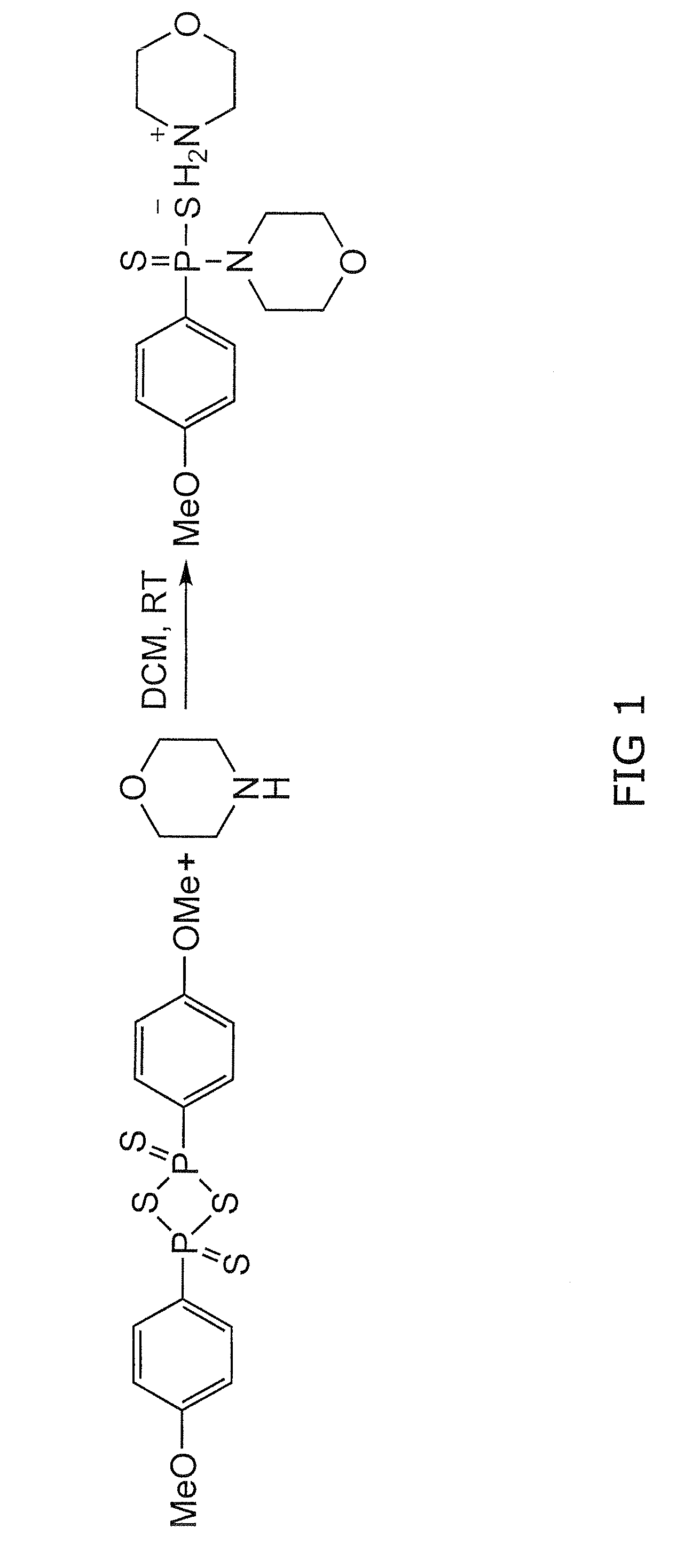
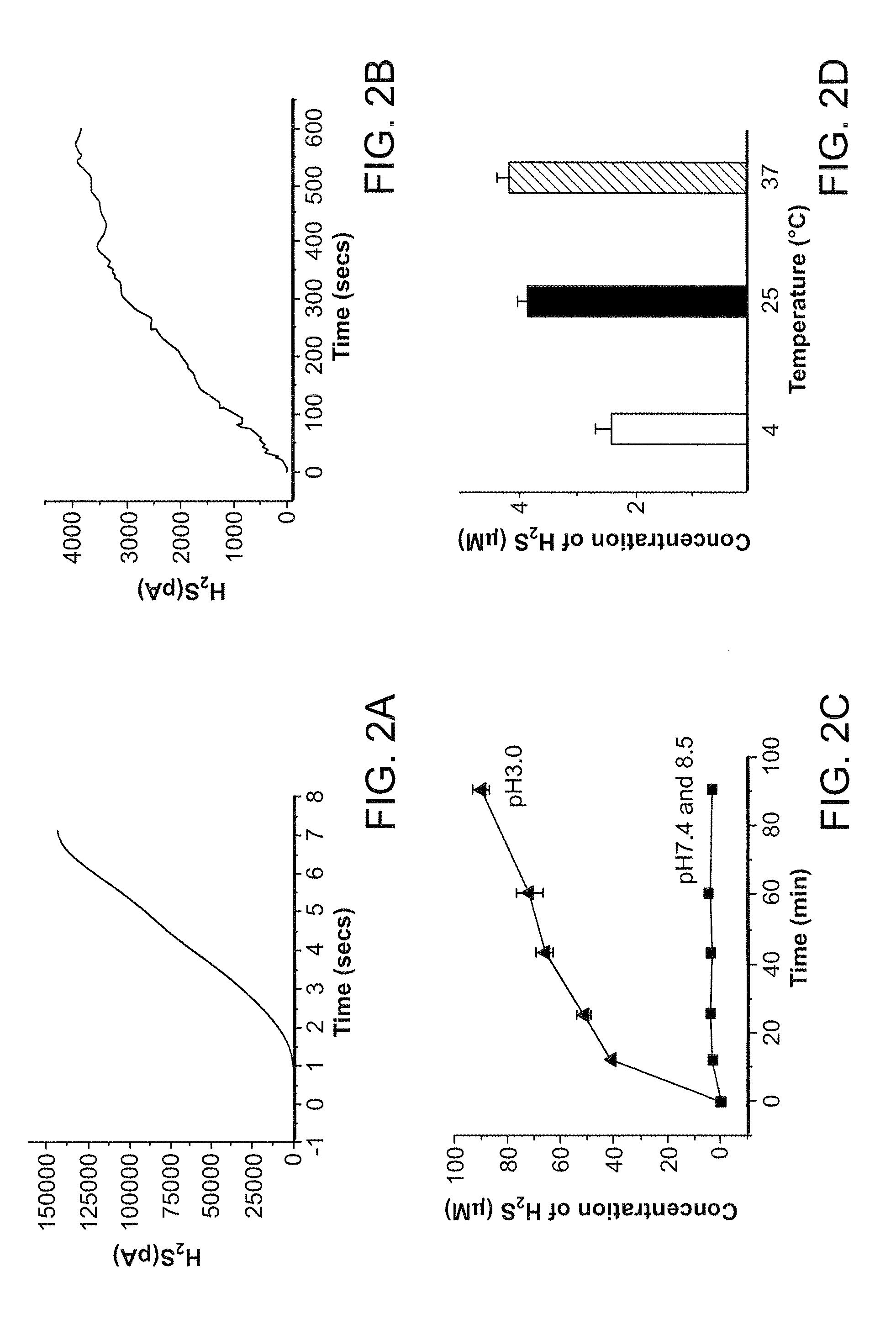
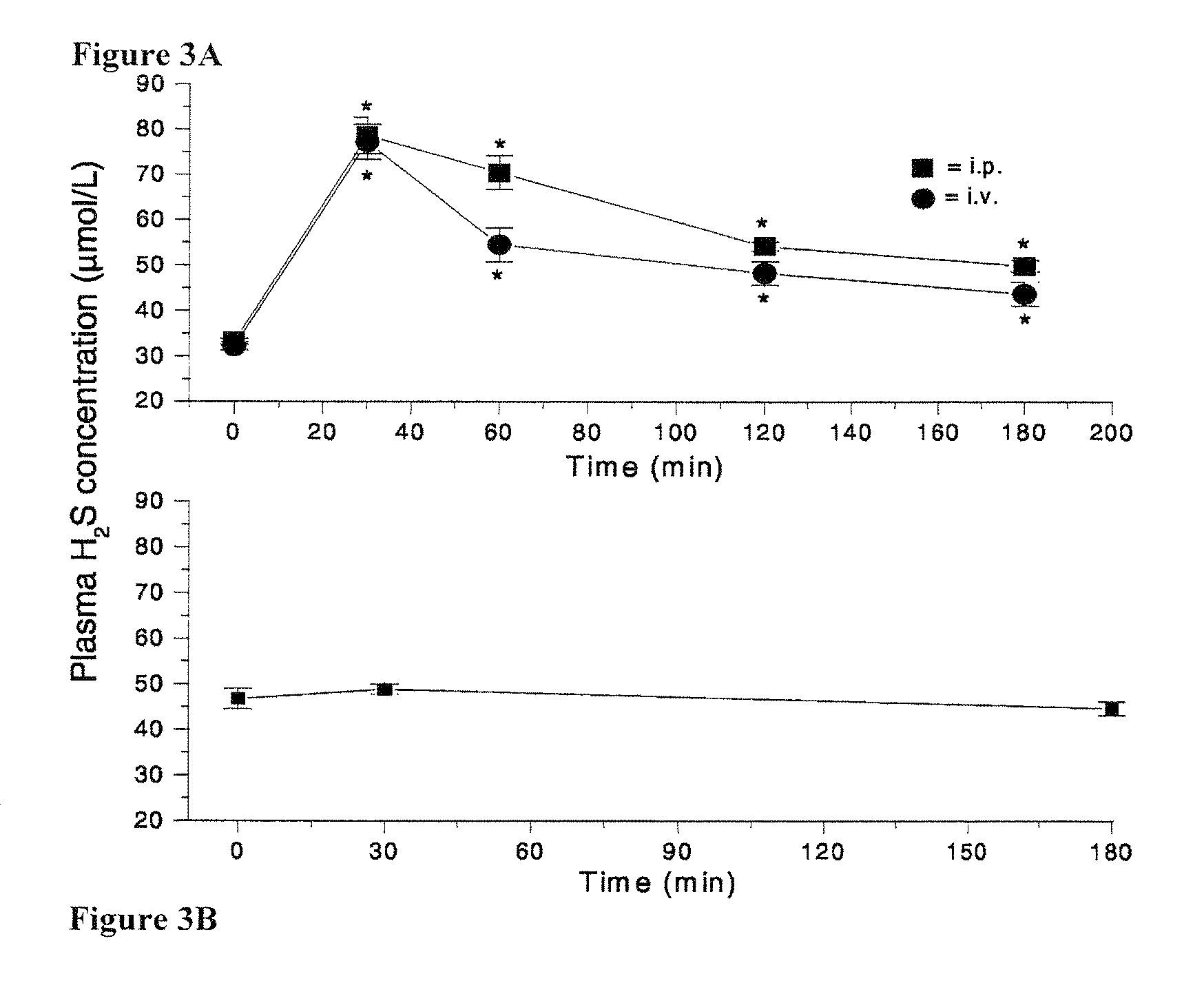
Morpholin-4-ium 4 methoxyphenyl (morpholino) phosphinodithioate (gyy4137) as a novel vasodilator agent
InactiveUS20100273743A1Lower blood pressureBiocidePhosphorous compound active ingredientsPharmaceutical medicineStructural formula
The invention is directed to a method of administering hydrogen sulfide (H2S) slowly and sustainably to an individual in need thereof comprising administering an effective amount of a compound represented by the following structural formula:or a pharmaceutically acceptable salt thereof.
Owner:NAT UNIV OF SINGAPORE
Compositions for reducing the deleterious effects of stress and aging
InactiveUS20120045426A1Reduce the amount requiredIncreasing tissue perfusionOrganic active ingredientsDipeptide ingredientsAdjuvantMedicine
The invention provides a formulation for treating stress and lessening fatigue. The formulation can be combined with water or another suitable liquid to provide a beverage for ease of administration. The formulation can include one or more of an energy compound, a vasodilator, a vasodilator adjuvant, and an antioxidant enhancer. In a typical formulation the energy compound is D-ribose or guanosine. The formulation can improve energy and alertness, and reduce the effects of stress and fatigue.
Owner:HYDROPEP
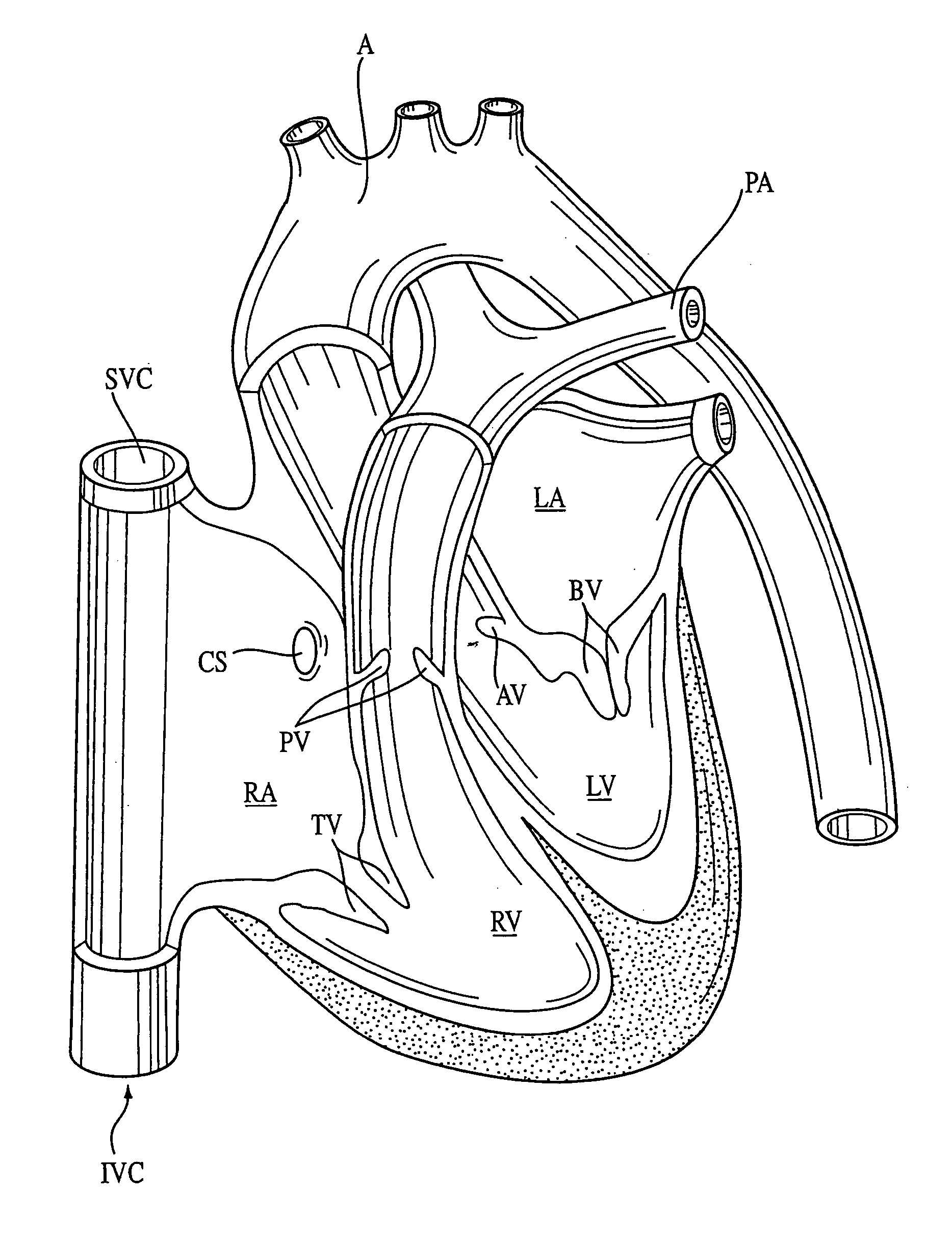
Compositions, kits, methods, and apparatus for transvascular delivery of a composition to an extravascular tissue of a mammal
Compositions, methods, kits, and apparatus are provided for delivering a macromolecular assembly such as a plasmid, virus vector, or other gene vector, to an extravascular tissue such as muscle tissue. The composition comprises the macromolecular assembly and a vascular permeability-enhancing agent. In another embodiment, the composition further comprises a vasodilating agent. The method of the invention comprises proving a vascular permeability-enhancing agent to a blood vessel and providing a macromolecular assembly to the vessel. An oxygenator useful for providing oxygen to a fluid extracorporeally prior to providing the fluid to a blood vessel of a mammal is included in the invention. Kits, apparatus, and methods for using the catheters described herein for isolating cardiac circulation, diverting caval blood flow from the right atrium, and for other purposes, are also described.
Owner:BRID CHARLES R +1
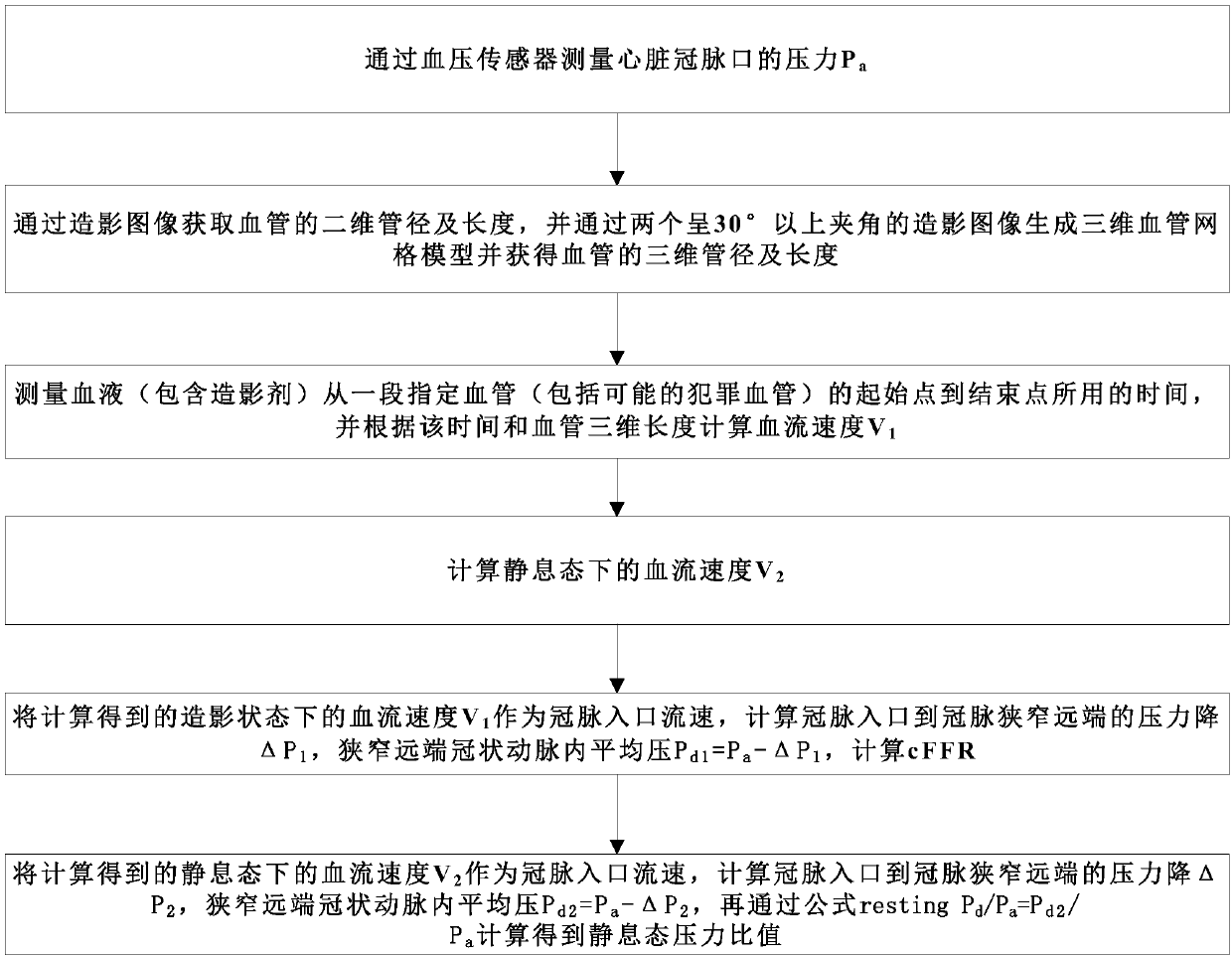
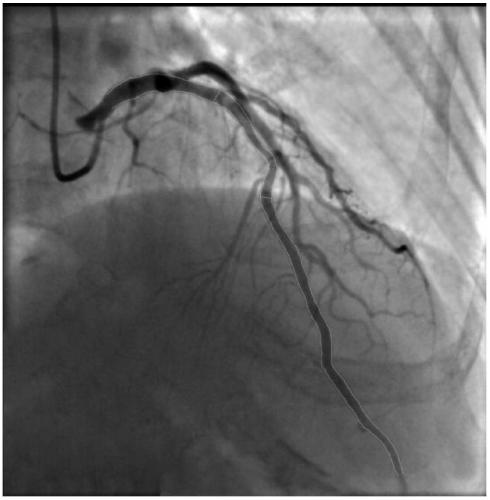
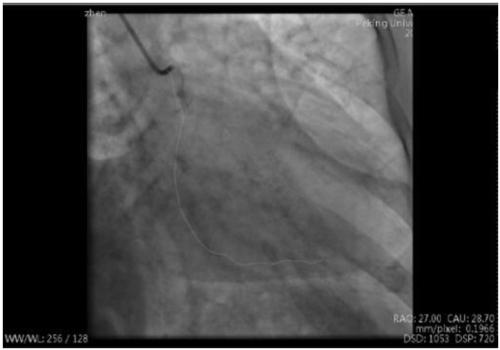
Method for calculating contrast blood flow reserve fraction and resting pressure ratio based on contrast images
PendingCN111166317ASimple and fast operationReduce the difficulty of surgeryImage analysisEvaluation of blood vesselsCoronary arteriesVasodilating Agent
The invention discloses a method for calculating contrast blood flow reserve fraction based on contrast images. The method comprises the following steps: measuring pressure Pa of a coronary artery port of a heart through a blood pressure sensor; acquiring two-dimensional pipe diameter and length of a blood vessel through the contrast images, generating a three-dimensional blood vessel grid model through two contrast images with included angles of 30 degrees or more, and acquiring the three-dimensional pipe diameter and length of the blood vessel; measuring time taken by blood containing contrast agents moving from a starting point to an ending point of a specified blood vessel, and calculating blood flow velocity V<1> according to the time and the three-dimensional length of the blood vessel; and taking the blood flow velocity V<1> as a coronary artery inlet flow velocity to calculate pressure drop delta P<1> from a coronary artery inlet to a coronary artery stenosis distal end, calculating average pressure P<d1> in the coronary artery stenosis distal end through a formula of P<d1>=Pa-delta P<1>, and calculating the contrast blood flow reserve fraction through a formula of cFFR=P<d1> / Pa. Therefore, cFFR and resuming Pd / Pa can be obtained by conventional contrast images without use of vasodilators.
Owner:SUZHOU RAINMED MEDICAL TECH CO LTD
Methods for treatment of pulmonary hypertension
ActiveUS10912778B2Improve exercise toleranceRelieve symptomsPowder deliveryDispersion deliveryPhosphodiesterase 5 inhibitorInhalation
Provided herein are methods for treating pulmonary hypertension. The methods include administering to a subject in need thereof an effective amount of a vasodilator, wherein the vasodilator is administered to the subject via inhalation pro re nata using a portable inhaler. In some embodiments, the vasodilator is a PDE5 inhibitor. Pharmaceutical compositions for pro re nata administration of vasodilators are also described.
Owner:RESPIRA THERAPEUTICS INC
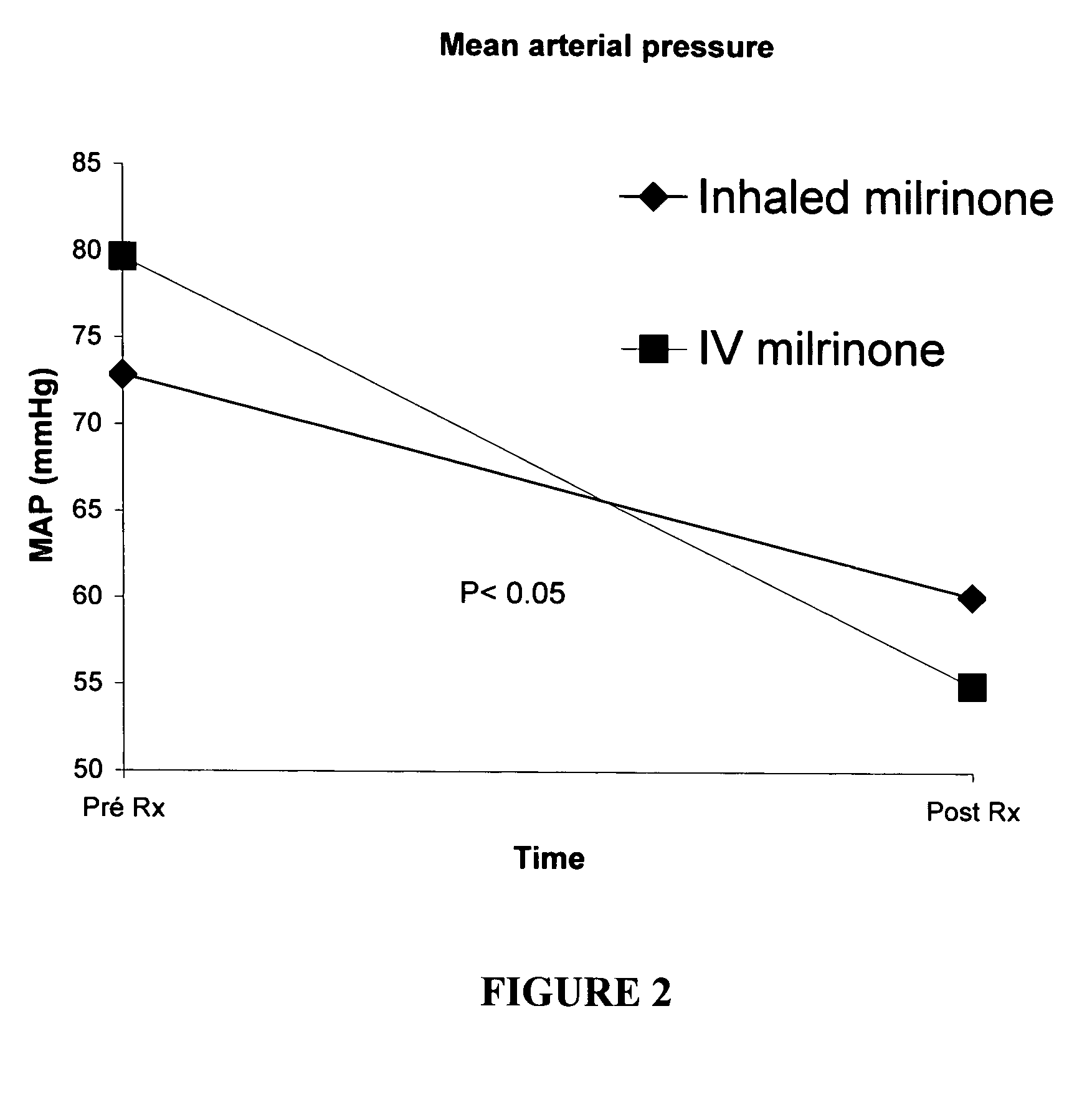
Method and substance for facilitating weaning, reducing morbidity and reducing mortality in cardiac surgeries involving extra-corporal circulation
InactiveUS20050049174A1Prevent right dysfunctionReducing right ventricular afterloadOrganic active ingredientsBiocideDobutamineVascular dilatation
Prophylactic strategies aimed at delivering vasodilators through inhalation in the pulmonary tree treat and prevent right ventricular dysfunction by reducing right ventricular afterload, facilitate separation from bypass and consequently decrease hemodynamic complications, morbidity and mortality. Examples of suitable vasodilatator include prostacyclin (flolan®), amrinone (inocor®), dobutamine (dobutrex®), nitroglycerine, nitroprussiate (nipruss®) and milrinone (primacor®).
Owner:INST DE CARDIOLOGIE DE MONTREAL
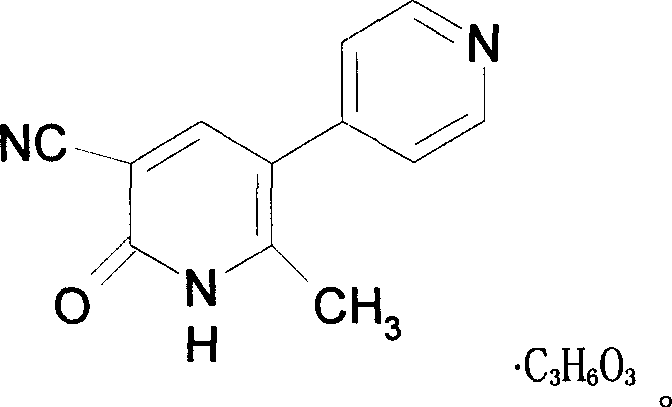
Milrinone lactate freezing-dried powder injection and preparation method thereof
InactiveCN101143144AImprove stabilityEasy to store and transportOrganic active ingredientsPowder deliveryBenzoic acidCellulose
A freeze-dry powder injection of lactic acid milrinone used for injecting and the preparation method of the injection belong to the medical technical field. The specification of the injection is 1 to 200mg (on the milrinone basis) of the lactic acid milrinone, which can be 5mg, 10mg, 20mg or 100mg etc., wherein one kind or more one kinds is of mannitol, low-molecular dextran, cyclodextrin, soluble starch, glucide matter, sodium chloride, benzoic acid, cellulose matter or water are selected as a carrier which is acceptable for the drug. The invention also relates to the method of preparing the freeze-dry powder injection of lactic acid milrinone, and the method includes the steps of preparing liquid medicine, removing pyrogen, removing bacterium, filling into a tank, freeze-drying etc., and white or nearly white loose block-shaped matter or powder of the product is obtained. The freeze-dry powder injection of the invention has good dissolubility, high biological utilization degree and good stability and ensures that the clinical use is safer and more convenient and can be used for vein dripping; the freeze-dry powder injection of the invention is applicable to the acute heart failure, the chronic heart failure, the intractable heart failure and the congestive heart failure which are caused by various reasons and have no effect or bad effect by the treatments of a digitalis, a diuretic and a vasodilator.
Owner:张刘森
Penis enlargement
InactiveUS20050065159A1Avoid prolonged useProlonged engorgement of a human penisBiocideElcosanoid active ingredientsVascular dilatationDiluent
A method for causing a permanent increase in the length and girth of a male subject's penis, the method comprising treatment comprising the step of (a) administering to the male an effective amount of a vasodilator selected from the group consisting of a vasodilator per se and compositions thereof comprising a pharmaceutically-acceptable diluent or carrier, to induce a cumulative prolonged engorgement of the subject's penis; and (b) repeating step (a) as necessary to cause the increase during the treatment. A potentiator which enhances the effect of the vasodilator may also be used.
Owner:DR KENNETH ADAMS MEDICINE PROFESSIONAL CORPORATIO
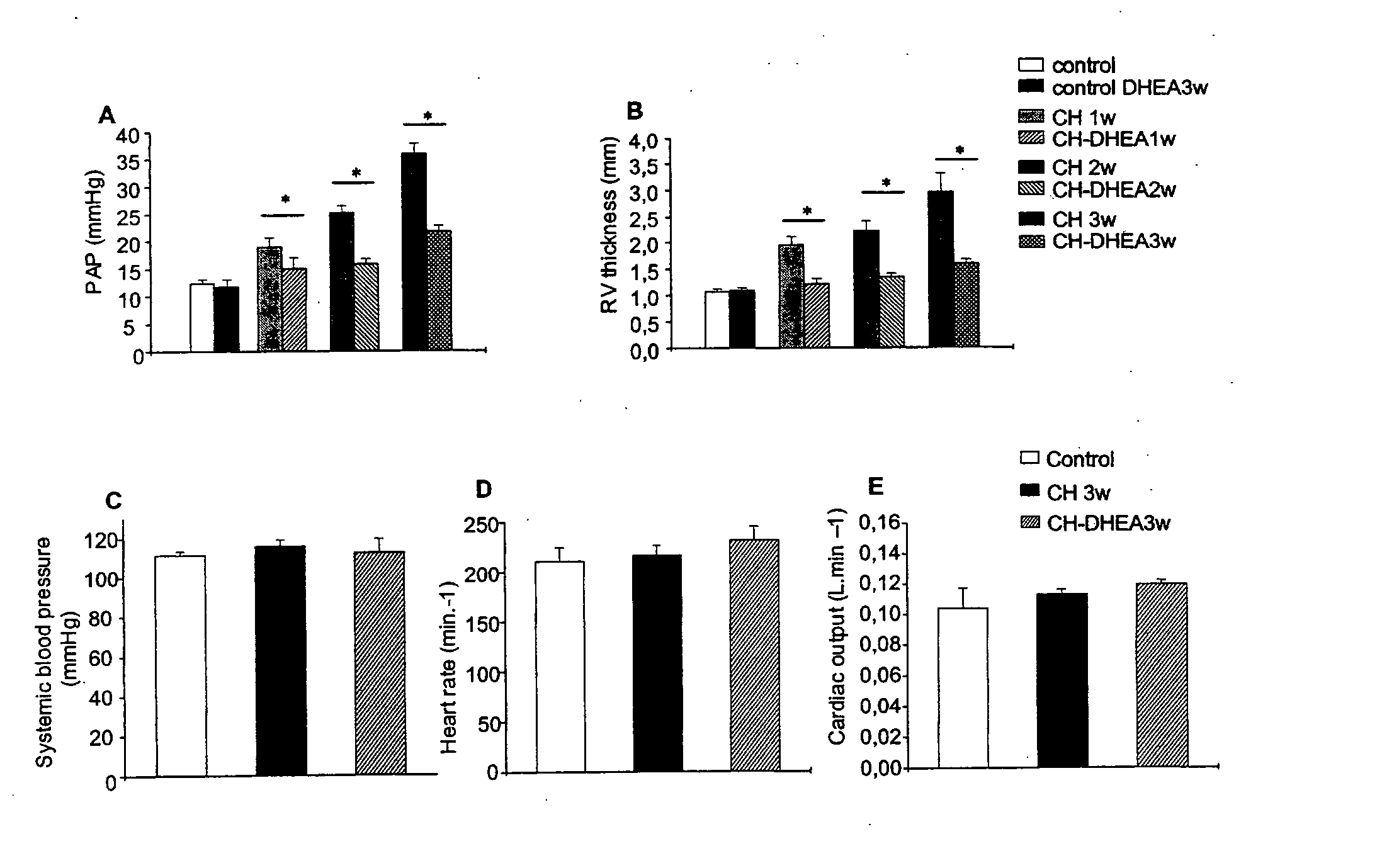
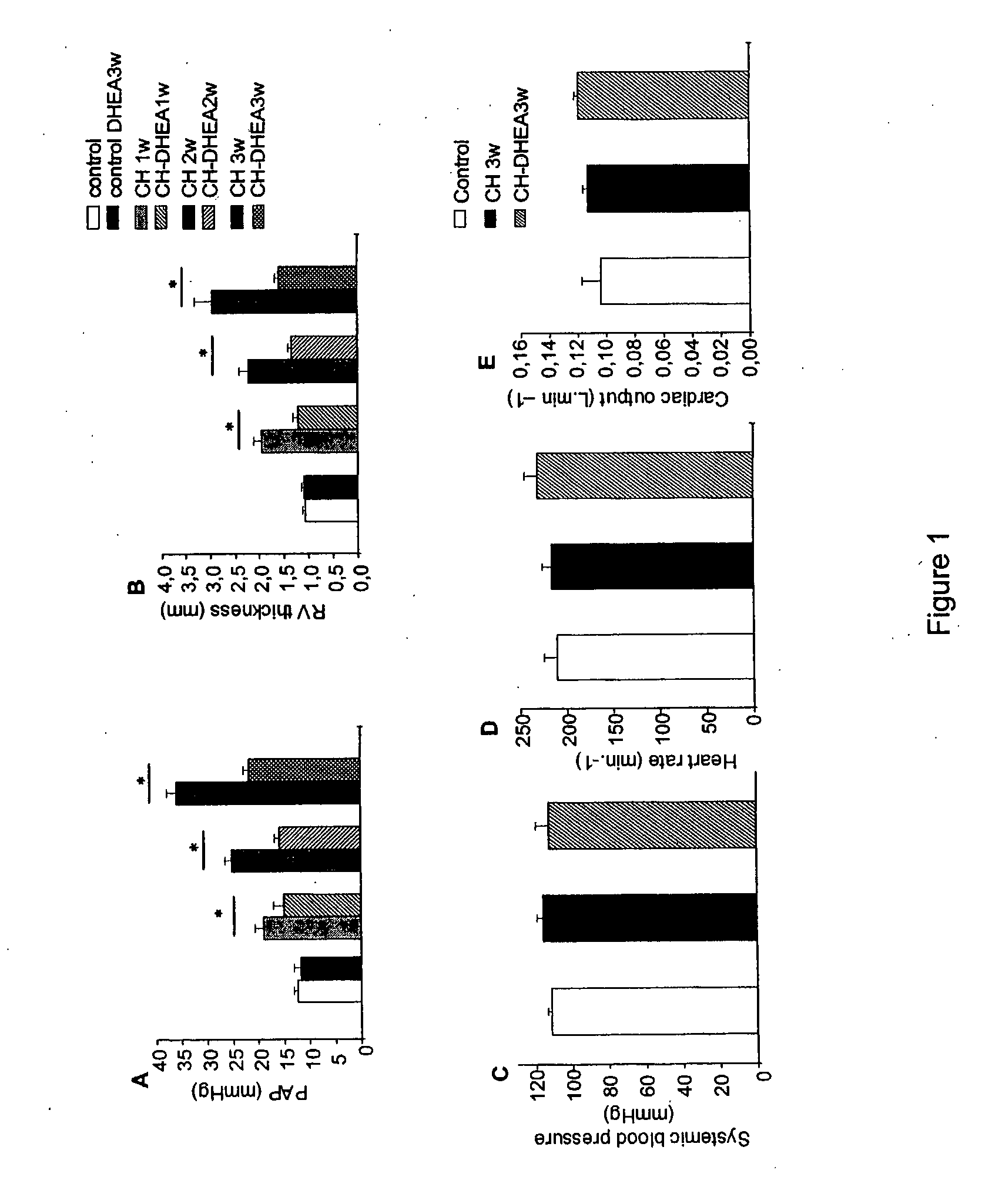
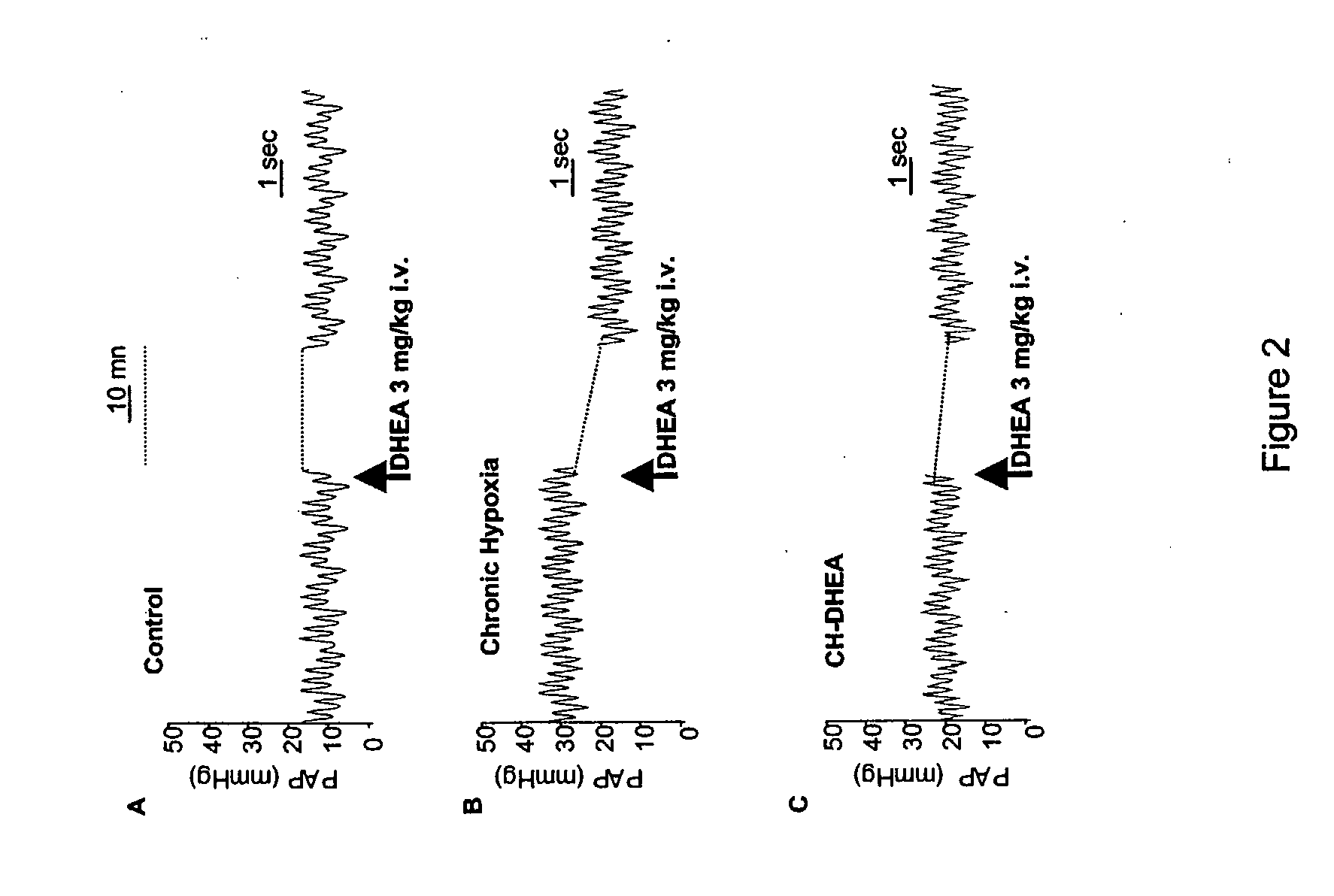
Treatment of Pulmonary Artery Hypertension with Dhea, Dheas, Dhea Analogs, or Dhea Derivatives
The present invention is related to the treatment and prevention of pulmonary vascular diseases. Administration of dehydroepiandrosterone (DHEA) has been found to prevent and decrease pulmonary artery hypertension. Accordingly, the invention discloses methods of treating or preventing pulmonary vascular diseases such as pulmonary artery hypertension by pulmonary administration of compositions containing DHEA, DHEAS, DHEA analogs, or DHEA derivatives. Additionally, the DHEA, DHEAS, DHEA analogs, or DHEA derivatives may be used in combination with other pharmaceutical agents, such as bronchodilators, vasodilators, anti-inflammatory agents, and anti-infectious agents to treat pulmonary diseases.
Owner:BAULIEU ETIENNE EMILE +1
Morpholin-4-ium 4 methoxyphenyl (morpholino) phosphinodithioate (GYY4137) as a novel vasodilator agent
InactiveUS8541396B2BiocidePhosphorous compound active ingredientsCompound (substance)Vasodilating Agent
The invention is directed to a method of administering hydrogen sulfide (H2S) slowly and sustainably to an individual in need thereof comprising administering an effective amount of a compound represented by the following structural formula:or a pharmaceutically acceptable salt thereof.
Owner:NAT UNIV OF SINGAPORE
Transdermal delivery of optical, spect, multimodal, drug or biological cargo laden nanoparticle(s) in small animals or humans
InactiveUS20090280064A1Uniformity of dose deliveryShorten the timeUltrasonic/sonic/infrasonic diagnosticsPowder deliveryMicro-needleMultiple layer
A method and a device are disclosed for transdermal delivery to an animal or human of biological cargo-laden nanoparticles. The particles may include multimodal optical molecular imaging probes. The particles may be delivered by providing them in a form that can be absorbed through the skin and applying them to the skin of an animal or human. The application may be accomplished using biological cargo-laden nanoparticles in a device attachable to the skin. The device may be attached directly to the skin by a device containing a vasodilating agent or agents, or micro needles, or multi-layer time release material. The biological cargo-laden nanoparticles may comprise drugs, vaccines, bio-pharmaceuticals, imaging contrast agents, multimodal imaging contrast agents, biomolecules, or anti-infectives. The device may include a first plurality of different types of biological cargo-laden nanoparticles located in a corresponding second plurality of separate time release layers.
Owner:BRUKER BIOSPIN
Compsns. for increasing energy i(in vivo)
The invention discloses precursors of adenosine triphosphate are administered orally to increase intracellular ATP concentration as dietary supplements or for treatment of reduced energy availability resulting from strenuous physical activity, illness or trauma. Pentose sugars are administered individually, mixed into dry food or in solution. The preferred pentose is D-ribose, singly or combined with creatine, pyruvate, L-carnitine and / or vasodilating agents. Additionally, magnesium, electrolytes, fatty acids and hexose sugars can be used. The compositions and methods of this invention are especially beneficial to mammals having reduced energy availability or high energy demand.
Owner:BIOENERGY INC
Transdermal drug delivery formulations and method of determining optimal amounts of vasodilators therein
InactiveUS20060013769A1Maximize efficiencyEasy to transportCompounds screening/testingOrganic active ingredientsVascular dilatationWhole body
A method for determining and demonstrating the role of vasodilator chemical agents in the development and practice of transdermal drug delivery systems. Vasodilator chemicals applied topically dilate the blood vessels in the skin tissue, which have been shown to facilitate or inhibit systemic or skin tissue deposition of drug substances. The level of stimulation and / or inhibition has been found to be dependent on the concentration and the identity of the specific vasodilator chemical(s) used as well as the drug molecule(s) to be delivered. This work teaches the need to consider specific formulation requirements when dealing with vasodilator chemicals for the creation of successful delivery vehicles in the transdermal drug delivery system. These requirements for very low concentrations of vasodilators were an unexpected and a surprise finding, in contrast to the concentrations of the vasodilators typically used to elicit an increase in skin blood flow.
Owner:BIOCHEMICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com