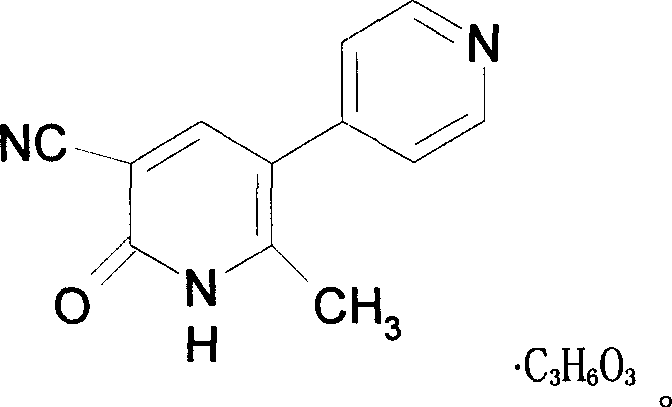
Milrinone lactate freezing-dried powder injection and preparation method thereof
A technology of milrinone lactate and needle injections, which is applied in freeze-dried delivery, powder delivery, pharmaceutical formulations, etc. It can solve problems that affect product safety and effective use, short valid period of use, short storage time, etc., and reduce clinical adverse reactions And side effects, enhanced stability, safe and convenient clinical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Preparation of milrinone lactate freeze-dried powder injection
[0035] Milrinone Lactate 20g
[0036] Mannitol 120g
[0037] Water for injection 4000ml
[0038] Made into 1000 bottles of freeze-dried product
[0039] Dosing: Weigh the mannitol that has been sterilized and depyrogenated in advance, and add water for injection to dissolve it. Then add milrinone lactic acid raw material for injection to dissolve, then add water for injection to a specified volume, such as 4000ml, so that the content of milrinone lactic acid is 0.005g / ml, and the content of mannitol is 0.03g / ml.
[0040] Pyrogen removal: add activated carbon for injection to the above medicinal solution (the amount of activated carbon is 0.05-0.3% of the weight of the medicinal solution), filter at room temperature for 30 minutes, and collect the filtrate.
[0041] Sterilization: The above-mentioned filtrate is subjected to positive pressure sterilization filtration with a sterile filter ...
Embodiment 2
[0045] Embodiment 2: Preparation of milrinone lactate freeze-dried powder injection
[0046] Milrinone Lactate 20g
[0047] Low Molecular Dextran 120g
[0048] Water for injection 4000ml
[0049] A total of 1000 bottles of freeze-dried products were made
[0050] The preparation method is the same as in Example 1, except that the auxiliary materials added are different. Example 3: Preparation of milrinone lactate freeze-dried powder injection
[0051] Milrinone Lactate 20g
[0052] Mannitol 60g
[0053] Low Molecular Dextran 60g
[0054] Water for injection 4000ml
[0055] A total of 1000 bottles of freeze-dried products were made
[0056] The preparation method is the same as in Example 1 except that the auxiliary materials added are different. The preparation method is the same as in Example 1 except that the auxiliary materials added are different.
Embodiment 4
[0057] Embodiment 4: Preparation of milrinone lactate freeze-dried powder injection
[0058] Milrinone Lactate 20g
[0059] Glycine 120g
[0060] Water for injection 4000ml
[0061] A total of 1000 bottles of freeze-dried products were made
[0062] The preparation method is the same as that of Example 1, Example 5, except that the added auxiliary materials are different. Example 5: Preparation of Milrinone Lactic Acid Lyophilized Powder Injection
[0063] Milrinone Lactate 20g
[0064] Cyclodextrin 120g
[0065] Water for injection 4000ml
[0066] A total of 1000 bottles of freeze-dried products were made
[0067] The preparation method is the same as in Example 1 except that the auxiliary materials added are different.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com