Acetylcholinesterase-derived peptides and uses thereof
a technology of acetylcholinesterase and peptides, applied in the field of stem cell survival and expansion, can solve the problems of limited molecular pathway(s), incomplete understanding of the mechanisms responsible for initiating this adjustment, and limited capacity to stimulate proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Hydrocortisone Elevates ACHE Gene Expression in Hematopoietic Stem Cells
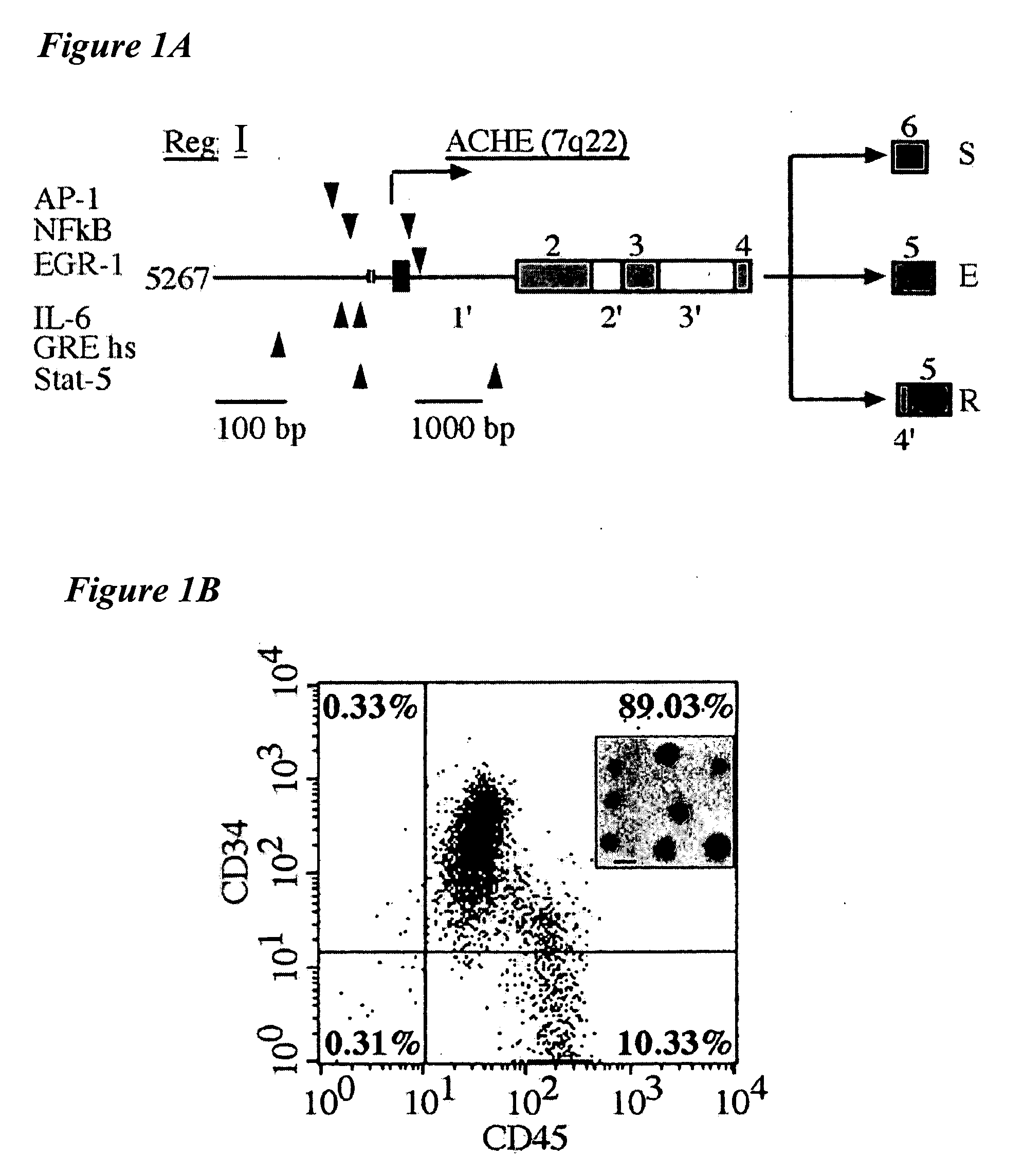
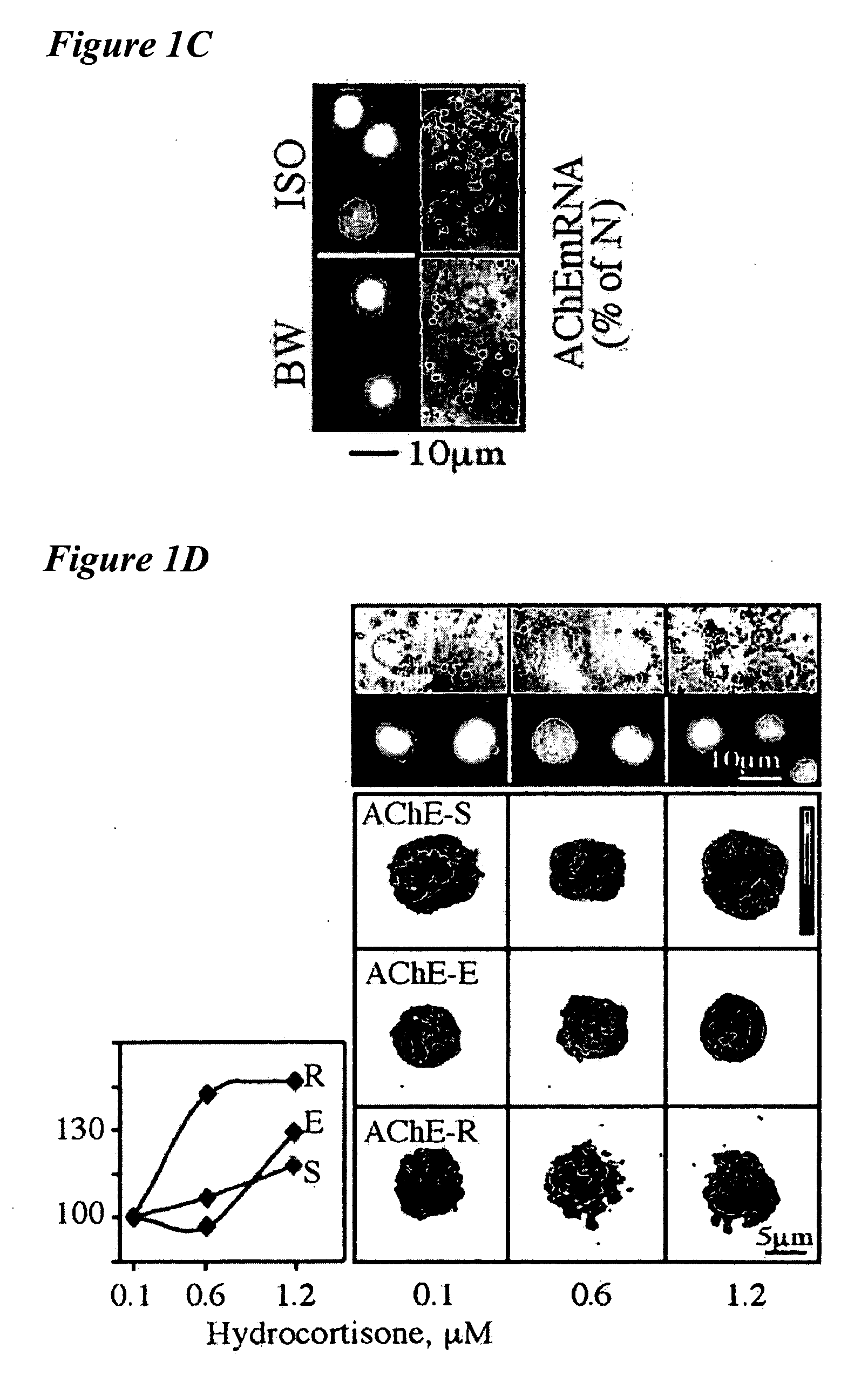
[0497] This example relates to ACHE gene expression in hematopoietic stem cells and the influence of hydrocortisone thereon. The inventors have searched the extended promoter of the human ACHE gene (cosmid accession no. AF002993) for consensus motifs that may bind stress-associated and hematopoietic transcription factors. Two such clusters, one located 17 Kb upstream from the transcription start site, and another positioned at the first intron, were found to include motifs for AP1, NFkB, EGR-1 (as identified by a matrix search against the TransFac database, Heinemeyer et al., Nucleic Acids Res. 26, 364-370, 1998), interleukin-6 (IL6), with the consensus sequence CTGGG / AAA, glucocorticoid responsive element (GRE) half palindromic site, TGTTCT, and Stat-5, TTCCCAGAA or TT(C / A)(C / T)N(A / G) (G / T)AA (FIG. 1A). Of these, the latter two motifs are known to be actively involved in hematopoiesis (Darnell et al., Science ...
example 2
Expression of ARP in CD34+ Cells
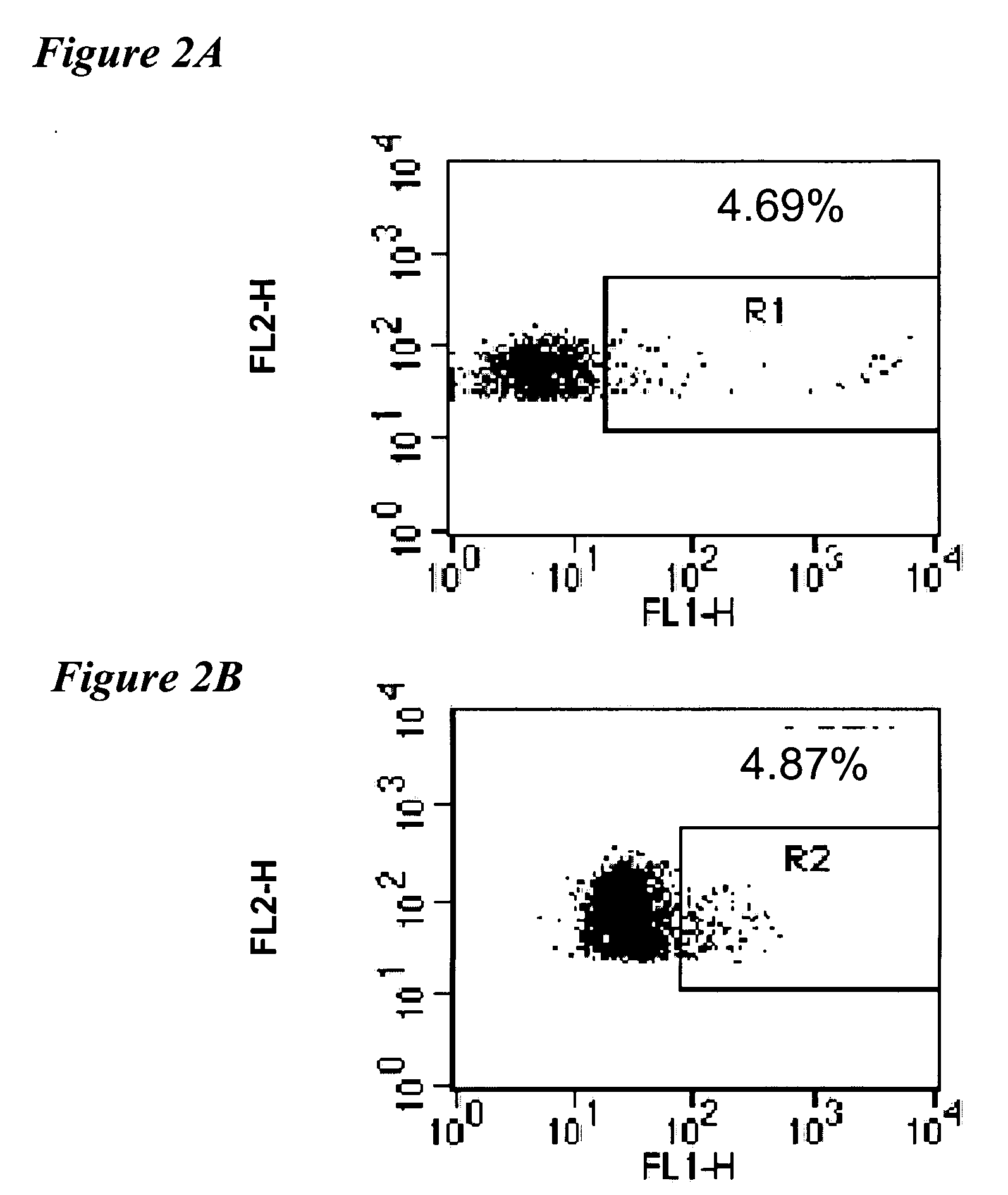
[0505] The expression of ARP in CD34+ hematopoietic cells was evaluated by flow cytometry in whole cord blood and bone marrow from a patient with immune thrombocytopenic purpura (ITP), as demonstrated in FIG. 2A and FIG. 2B respectively. Bone marrow from ITP patients was chosen to study ARP expression in hematopoietic progenitor cells due to the high turnover of normal CD34+ in these patients. Cells were fixed and permeabilized with Fix and Perm (Caltag, Calif., US) and stained with monoclonal antibodies to CD34 conjugated to pycoerythrin (Beckton Dickinson, California, US) indicated as FL-2 and with highly specific rabbit anti-ARP antibodies followed by anti rabbit antibodies conjugated to fluorescein isothiocyanate, expression indicated as percentage of positive cells.
[0506] These findings demonstrate higher expression of AChE-R in proliferating hematopoietic progenitors from either newborns or individuals suffering from over-proliferation of bloo...
example 3
Readthrough AChE is Overproduced in the Myeloidogenic Mid-gestation Liver
[0507] To study the relevance of each of the AChEmRNA transcripts during development of the hematopoietic organs, in situ hybridization was performed on paraffin-embedded sections taken from human fetuses at different gestational ages. Consistent with the embryonic spatiotemporal shifts in blood cell forming tissues, we observed changes in the labeling intensity with the various probes used, in the aorta-gonad-mesonephric region (AGM), liver, spleen and bone marrow cells. FIG. 3A schematically presents the migration of hematopoiesis between the various blood cell forming tissues during fetal development. The top left of the figure represents a sagital section of a human embryo showing the hematopoietic organs—AGM (aorta-gonad-mesonephros), LIV (liver), SPL (spleen), and BM (bone marrow). The top right of the figure is a scheme of gestational shifts in hematopoietic processes which shows the relative intensity ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com