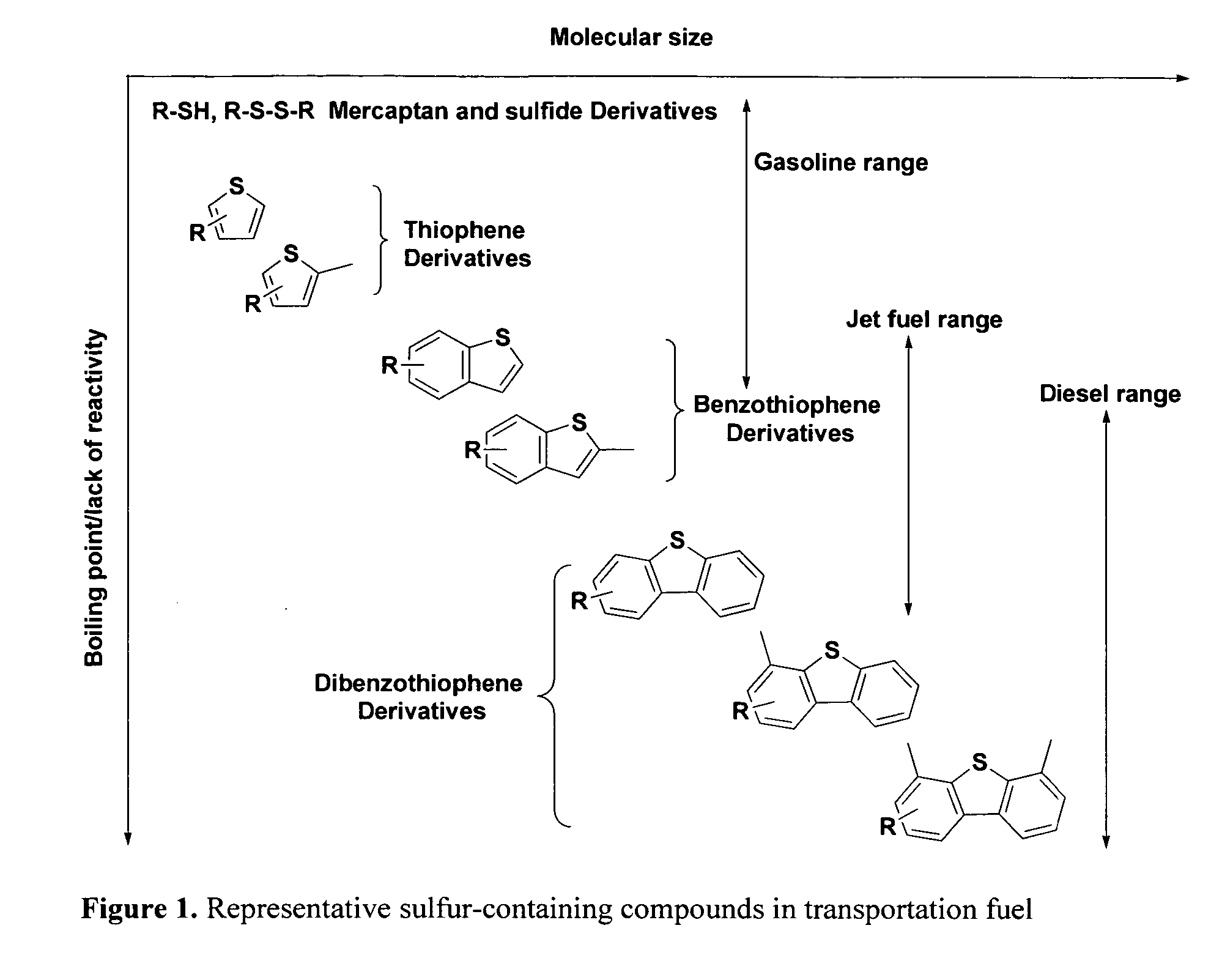
Sorbent compositions and desulfurization method using same
a technology of sorbent composition and desulfurization method, which is applied in the direction of hydrocarbon oil cracking, other chemical processes, chemistry apparatus and processes, etc., can solve the problems of no other fuel can match diesel in its ability to move freight economically, and the sulfur compounds in liquid hydrocarbon fuels have been linked to serious human health and environmental problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
working examples
[0031] (All Adsorption Experiments Have Been Replicated to Reach a Less than 5% Total Sulfur Adsorption Capacity Difference):
Preparation of Sorbent (Dry Premixing)
[0032] The preparation of iron (III) nitrate nonahydrate-doped Montmorillonite K10 clay is representative: iron (III) nitrate nonahydrate (Aldrich Chemical Co., Milwaukee, Wis.) (0.020 g) was contacted with Montmorillonite K10 clay (Aldrich Chemical Co., Milwaukee, Wis.) (0.200 g) in a mortar and pestle. Alternatively, they were physically mixed in a glass vial for batch adsorption tests. After thorough mixing at room temperature (25-30° C.), the sorbent was added to the jet fuel or diesel.
Alternative Method (Preferred)
[0033] 5 ml of model gasoline, jet fuel or diesel was added to 0.200 g of clay and 0.020-0.040 g of metal nitrate (10-20% metal nitrate loading) in a 15 mL capped sample vial equipped with a magnetic stirring bar using a 5.0 mL measuring pipette, the mixture stirred at room temperature (25-30° C.) for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com