Human BNP immunospecific antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
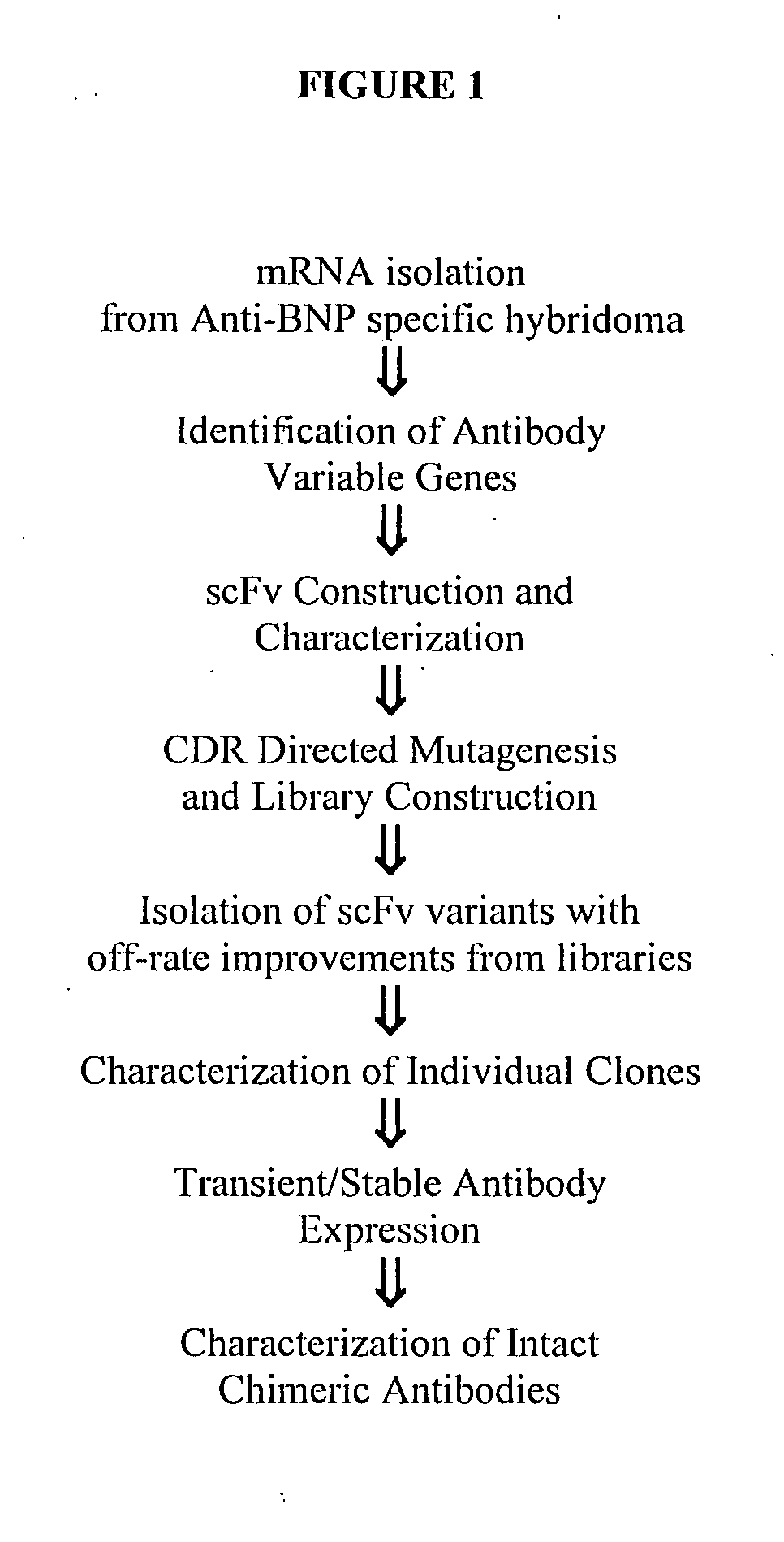
Identification of Immunoglobulin Genes
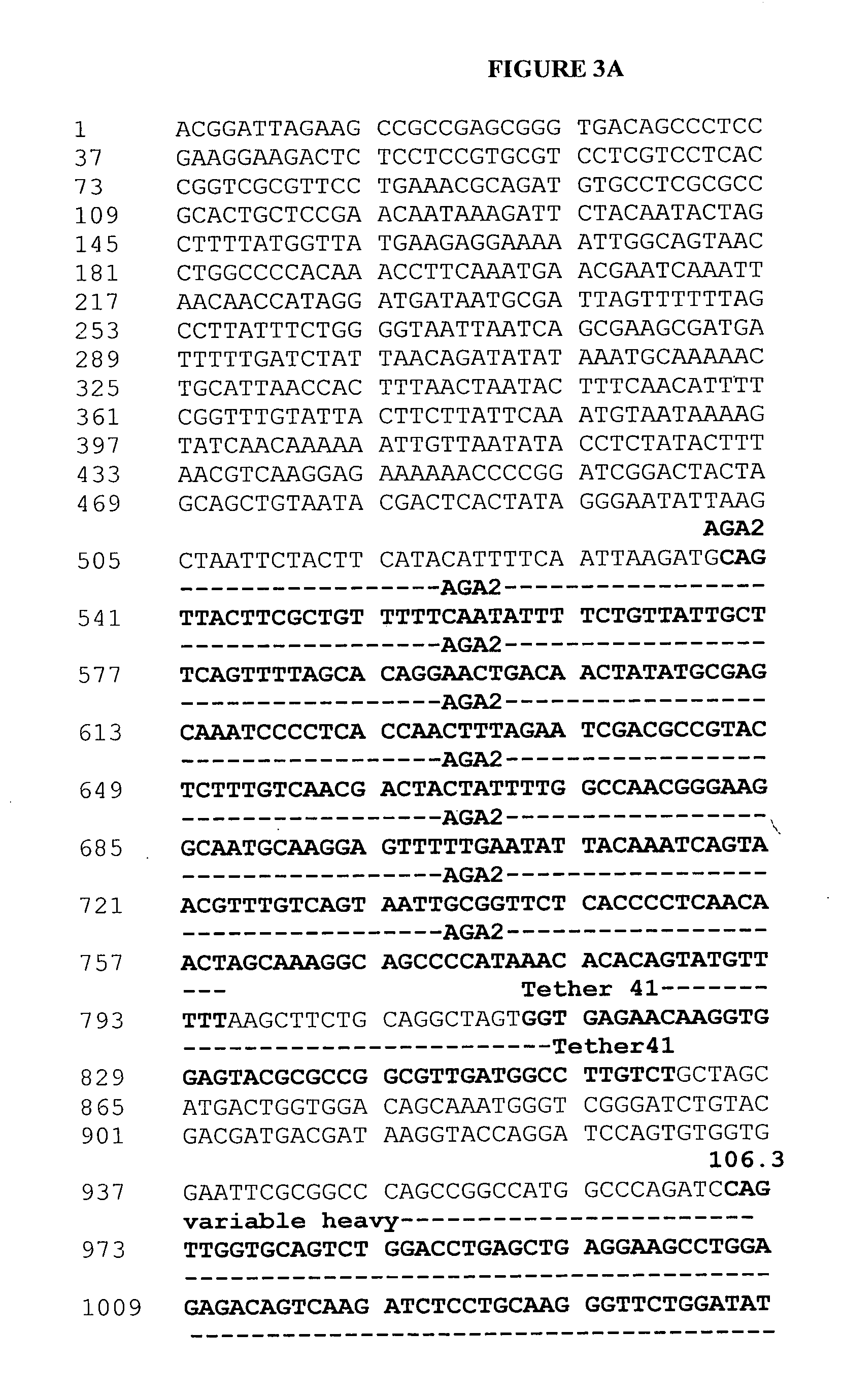
[0307] Messenger RNA was isolated from subcloned anti-BNP 106.3 hybridoma cells (hybridoma cell line 106.3 (A.T.C.C. Accession No. HB-12044) is described in U.S. Pat. No. 6,162,902). 106.3 mRNA was utilized in a reverse transcriptase-polymerase chain reaction using a mouse Ig primer set kit purchased from Novagen (Novagen (which is an Affiliate of Merck KGaA, Darmstadt, Germany), Cat No. 69831-3) with immunoglobulin gene specific primers contained in the kit. The resulting PCR products were sequenced and thus the immunoglobulin variable heavy and variable light chain genes were identified (See FIGS. 3A-3E and SEQ ID NO:1).
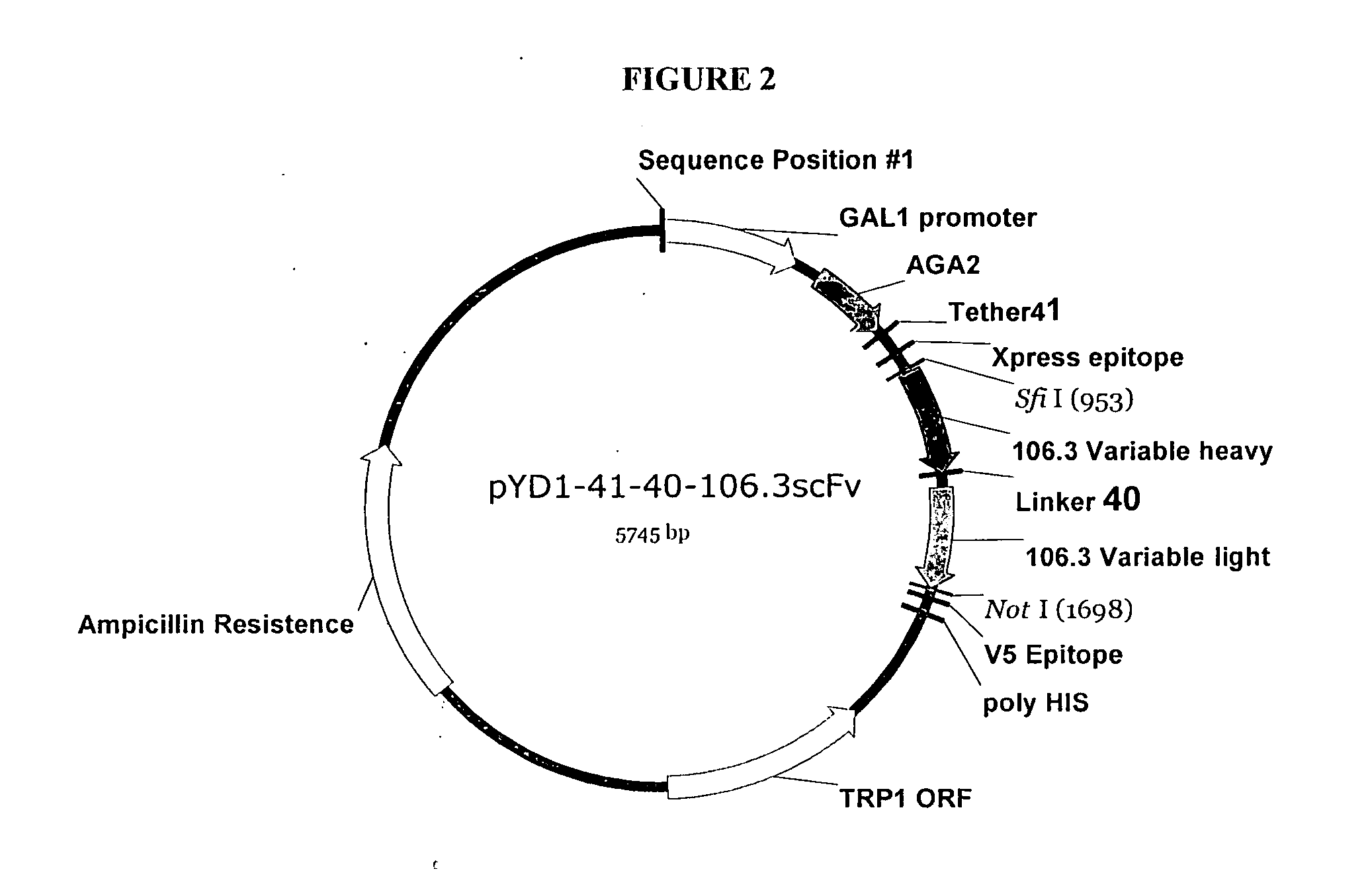
Cloning 106.3 Variable Region Genes into pYD41 Vector
[0308] A yeast display system was used to express unmutated anti-BNP proteins (described herein infra) and a library of anti-BNP proteins on the yeast surface as a fusion to the yeast protein AGA2. A yeast display vector called pYD (Invitrogen, Carlsbad, Calif.), was use...
example 2
ATCC Deposit Information
[0339] Chinese Hamster Ovary cell line for BNP106.3sc128am1CHO1162-236 was deposited with the American Type Culture Collection (hereinafter referred to as “A.T.C.C.”), 10801 University Blvd., Manassas, Va. 20110-2209, on Sep. 20, 2005 and assigned A.T.C.C. Accession No. PTA-6987.
example 3
Competitive Immunoassay Using a Single Antibody Format
[0340] The antibody produced by CHO cell line AM1 (“antibody AM1”) described above in Examples 1 and 2 was purified and tested to determine the antibody's ability to bind human cyclic BNP1-32 in a single antibody format on the ARCHITECT® instrument (Abbott Laboratories, Abbott Park, Ill. This instrument is described in U.S. Pat. No. 5,468,646). This single antibody format encompasses the use of only one analyte specific antibody in the testing reaction.
[0341] Paramagnetic microparticles (hereinafter “microparticles”, Polymer Labs, Amherst, Mass.) were washed and then reacted with serially diluted Goat anti-human antibody (Jackson ImmunoResearch, West Grove, Pa.). The Goat anti-human antibody was coated onto the paramagnetic microparticles using the techniques described in U.S. Pat. No. 6,162,902. Specifically, EDAC coupling was used (EDAC is generally used as a carboxyl activating agent for amide bonding with primary amines. In...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Frequency | aaaaa | aaaaa |
| Frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com