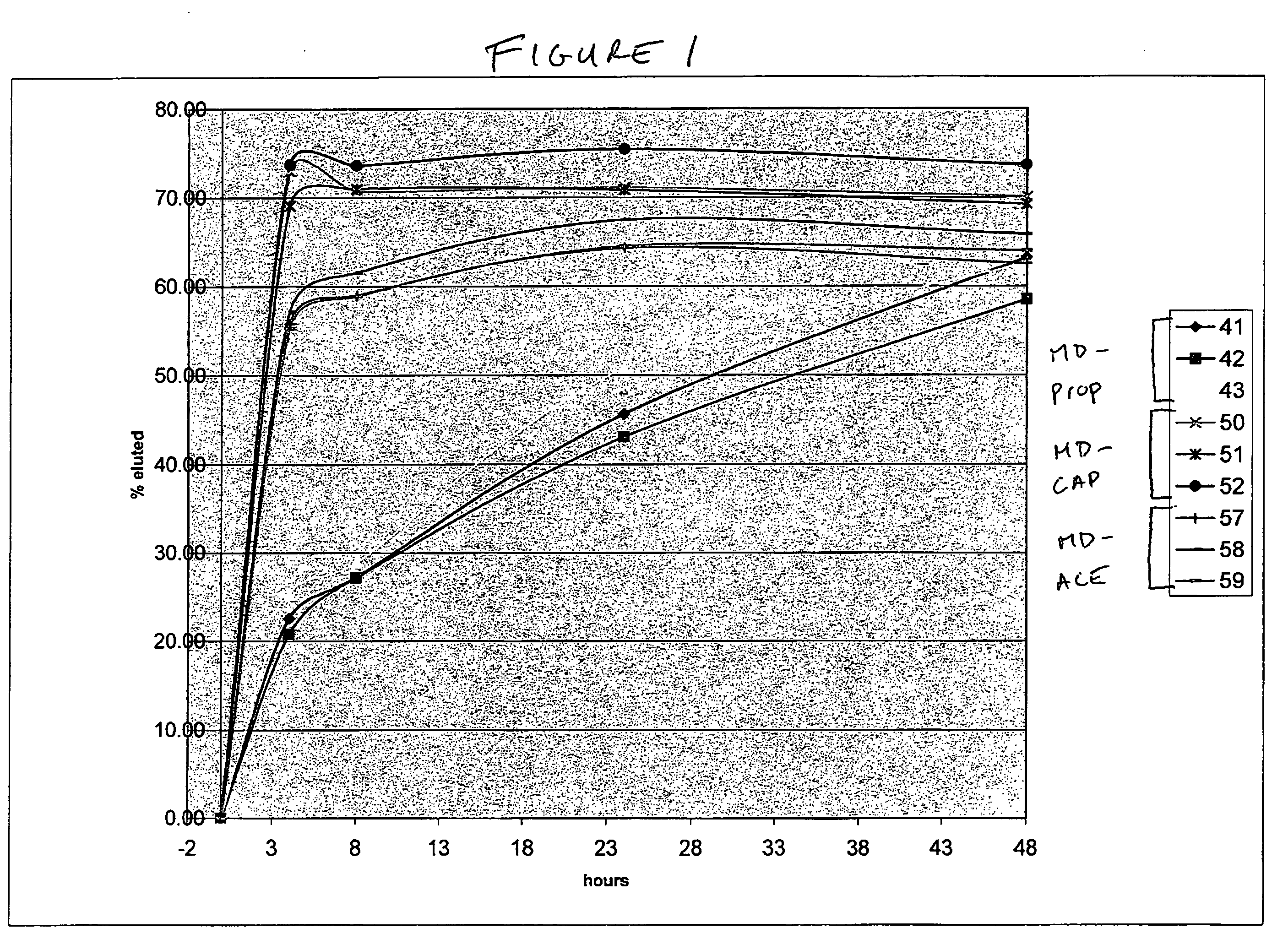
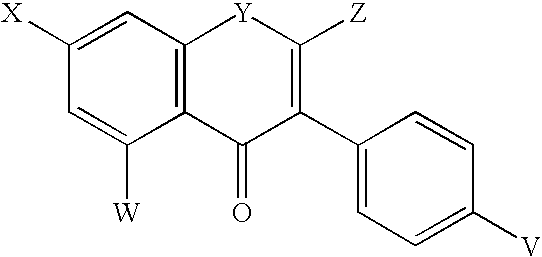
Biodegradable hydrophobic polysaccharide-based coatings
a technology of hydrophobic polysaccharide and biodegradable coating, which is applied in the field of biodegradable coatings, can solve the problems of increasing the risk of complications, patient discomfort, and limitations of drug injection, and achieves the effects of prolonging the in vivo life, slowing down the erosion or degradation of the structural portion, and improving the biocompatibility of the articl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0218]11 g of dried maltodextrin (GPC, Grain Processing Corporation, Muscatine, Iowa) was dissolved in 100 mls of dimethyl sulfoxide with stirring. When the solution was complete, 20 g (0.244 moles, 19.32 mls, Sigma-Aldrich) of 1-methylimidizole followed by 50 g (0.32 moles, 52 mls, Sigma-Aldrich, Milwaukee, Wis.) of butyric anhydride were added with stirring at room temperature. The reaction solution was stirred for one hour and was then quenched with deionized water. The taffy-like material that precipitated from the quenched reaction mixture was placed in 1,000 MWCO dialysis tubing and dialyzed vs. continuous flow deionized water for three days. After this time the solid product was lyophilized. 23.169 g of a white powdery solid was obtained. The theoretical degree of substitution (DS) was 2.5.
example 2
[0219]10 g of dried MD was dissolved in 100 mls of dimethyl sulfoxide with stirring. When the solution was complete, 23.7 g (0.29 moles, 22.9 mls) of 1-methylimidizole followed by 29.34 g (0.29 moles, 27.16 mls) of acetic anhydride (Sigma-Aldrich, Milwaukee, Wis.) were added with stirring at room temperature. The reaction solution was stirred for one hour and was then slowly add to 750 mls of deionized water in a Waring blender. The precipitated solid was collected via filtration, re-suspended in 1 L of deionized water and stirred for one hour. The solid was collected via filtration and dried in vacuo. 15.92 g of a yellow powdery solid was obtained. The theoretical DS was 2.5
example 3
[0220]10 g of dried MD was dissolved in 100 mls of dimethyl sulfoxide with stirring. When the solution was complete, 9.49 g (0.11 moles, 9.17 mls) of 1-methylimidizole followed by 18.19 g (0.11 moles, 18.81 mls) of butyric anhydride were added with stirring at room temperature. The reaction solution was stirred for one hour and was then slowly add to 750 mls of deionized water in a Waring blender. The precipitated solid was collected via filtration, re-suspended in 1 L of deionized water and stirred for one hour. The solid was collected via filtration and dried in vacuo. 16.11 g of a white powdery solid was obtained. The theoretical DS was 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com