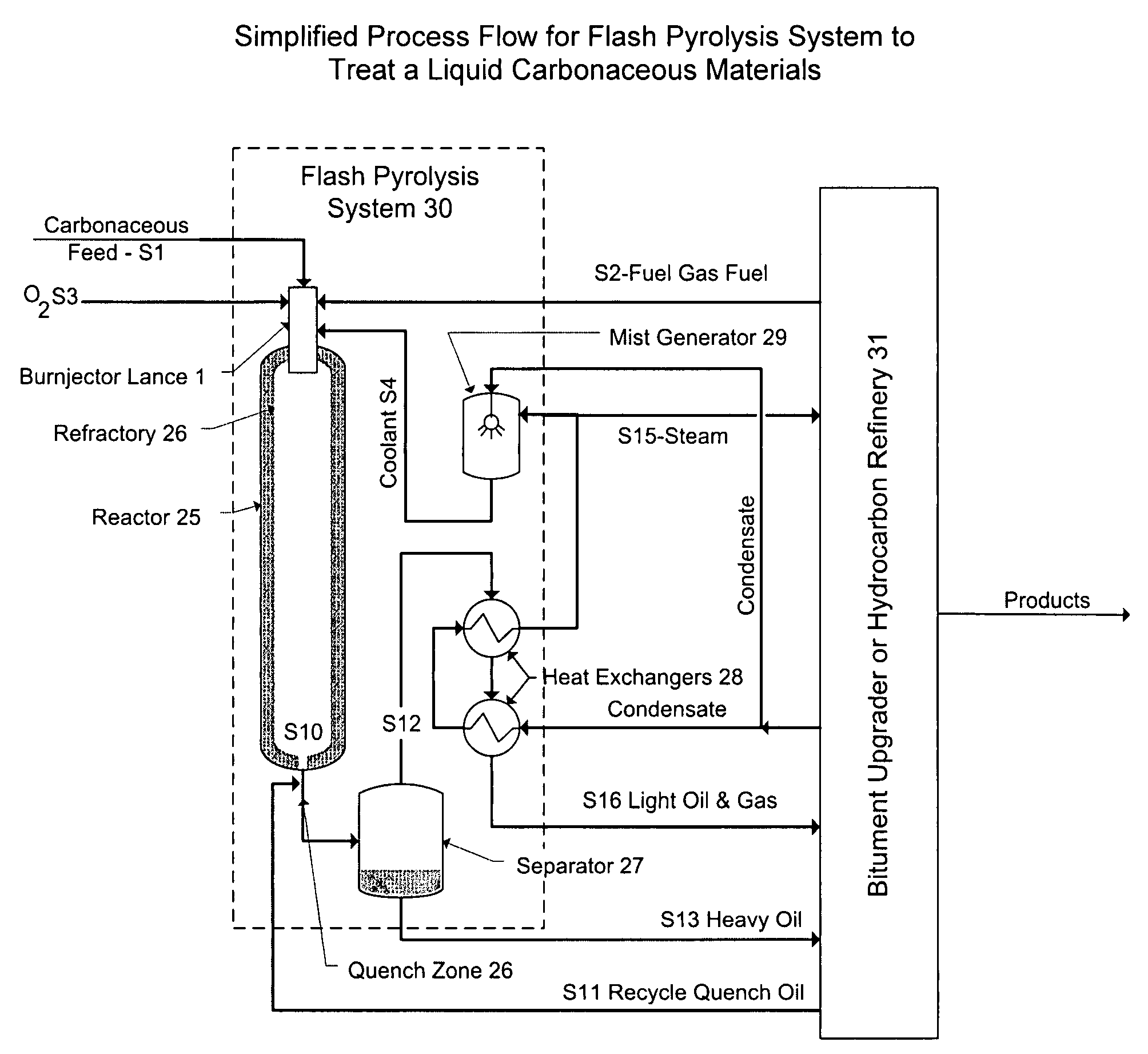
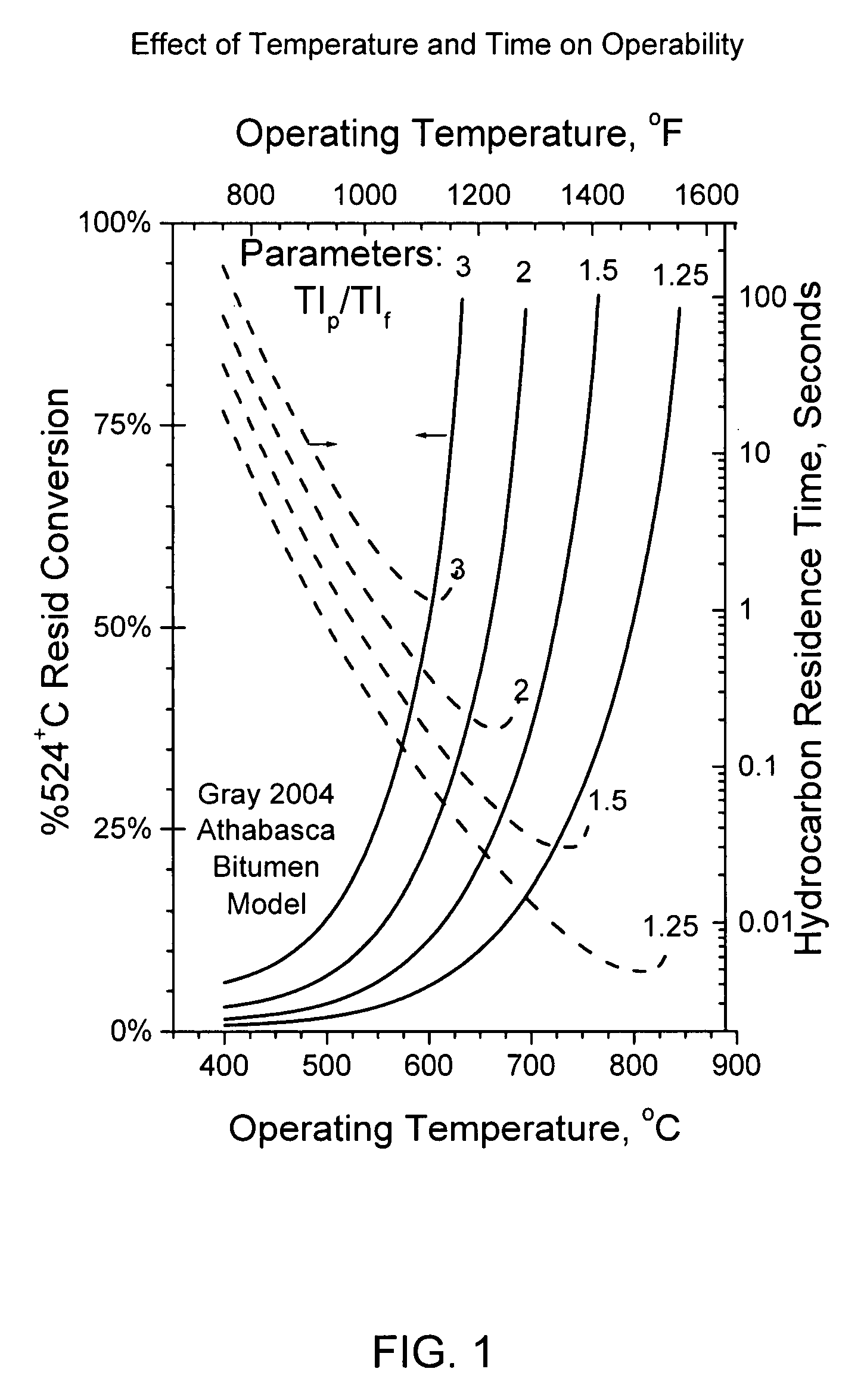
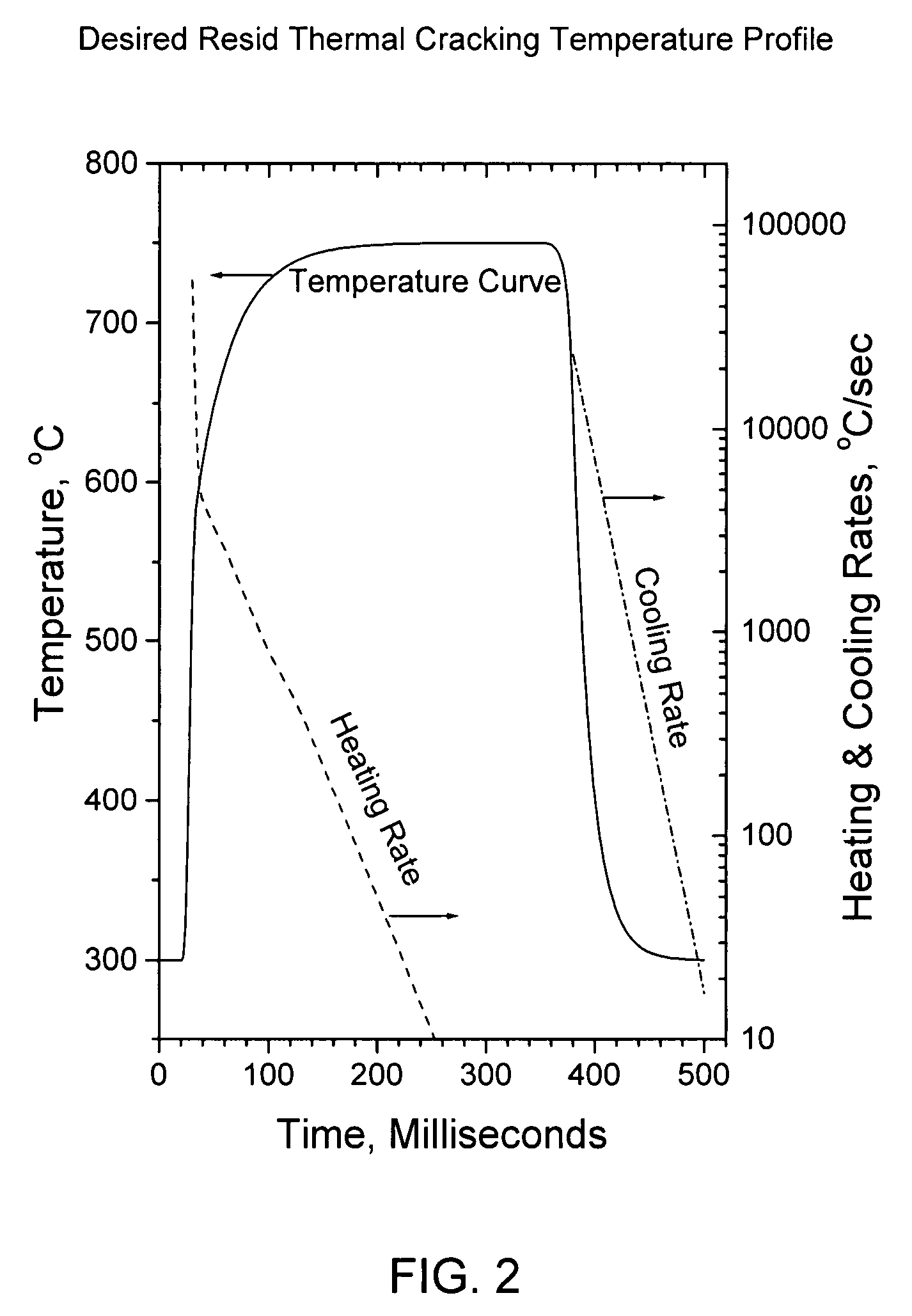
Flash pyrolosis method for carbonaceous materials
a carbonaceous material and flash pyrolysis technology, applied in the direction of combustible gas production, thermal non-catalytic cracking, discharging devices, etc., can solve the problem of limited resid conversion in thermal cracker and delayed coker unit operations, the coke product is much more difficult to grind and burn than the delayed coke product, and achieves rapid cooling of the reaction products of the lower boiling poin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
[0049]Table 1 summarizes the vacuum bitumen resid feed properties. Table 2 presented below shows a component material balance for flash pyrolysis system treating 10,000 barrels per day of the bitumen in Table 2.
TABLE 2Example Component Heat & Material BalanceStream12345678910T, ° C.315350252153,2922751,8781,171315710P, bar20.020.020.021.020.00.220.02.02.02.0PhaseLiquidGasGasGas-LiquidGasGas-LiquidGasGasLiquidGasEnergy, MJ / hr1Thermal52,036−6,2890−388,512−6,289−385,277−394,801−477,79752,036−342,977Kinetic———————82,996—212Specie, Kg / hrCH4—6,961.4————————CO————644.2—1,484.9———CO2————18,084.8—16,763.919,096.9—19,096.9H————52.4—0.3———H2————225.5—59.5166.6—166.6H2O(l)———0.0——————H2O(v)———29,779.811,741.129,779.844,880.043,925.2—43,925.2HO————2,660.9—0.1———O2——26,447.5———————Cut 131,865.4———————31,865.41,851.1Cut 237,067.2———————37,067.28,672.2Cut 32,872.2———————2,872.230,238.6Cut 4—————————31,042.9Total71,804.86,961.426,447.529,779.833,408.929,779.863,188.763,188.771,804.8134,993.51Cut 1 a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| velocity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com