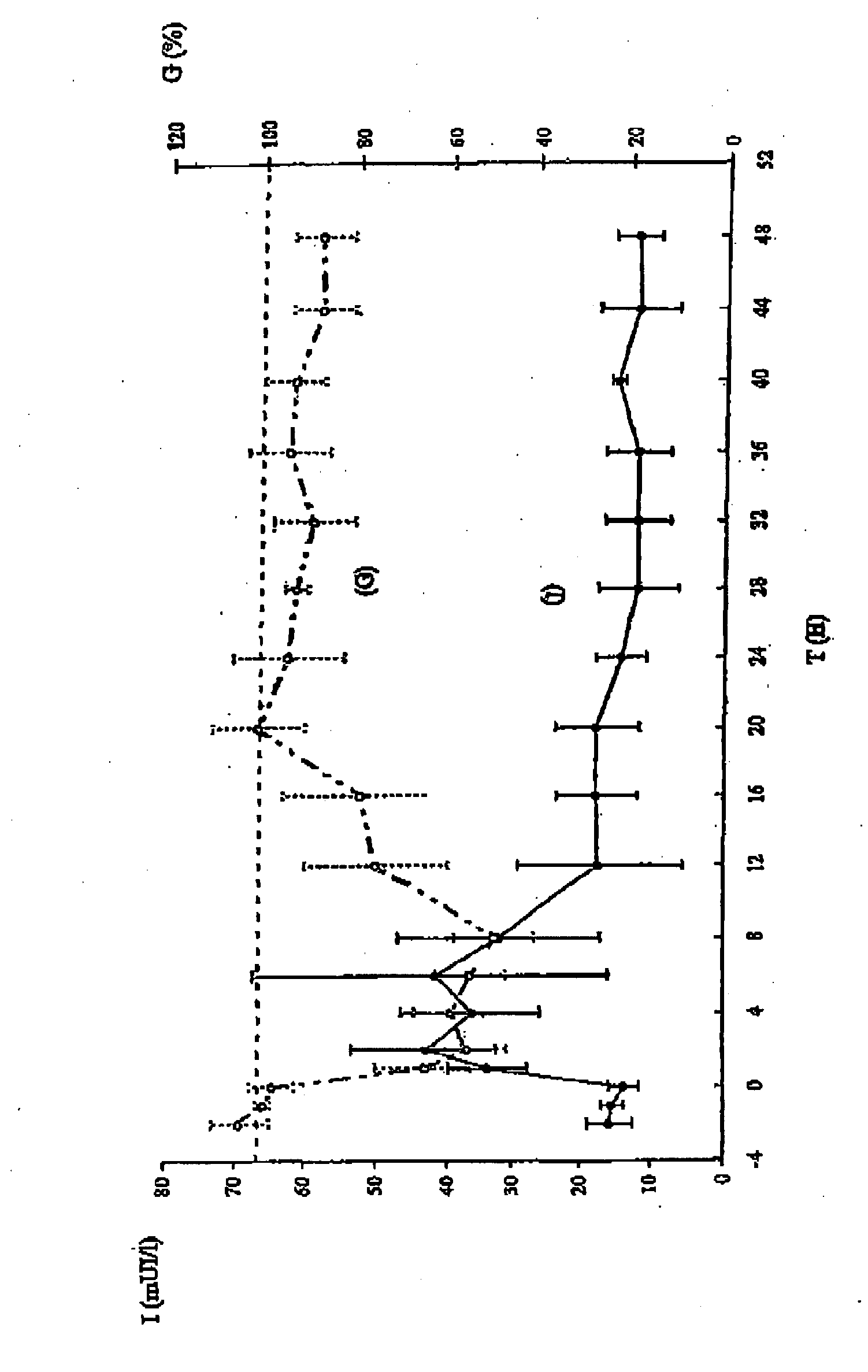
Colloidal suspension of submicronic particles for carrying active principles and their mode of preparation
a technology of carrier particles and colloidal suspensions, which is applied in the field of colloidal suspensions of carrier particles, can solve the problems of disclosing the use of copolymers, and achieve the effect of high bioavailability of ap and easy and economical production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Poly(Leucine / Methyl Glutamate) Block Copolymer
[0183] The techniques used to polymerize NCA to polymers with block or random structures are known to those skilled in the art and are described in detail in the work by H. R. KRICHELDORF entitled “α-Amino acid N-carboxy anhydrides and related heterocycles”, Springer Verlag (1987). The synthesis of one such polymer is specified below.
[0184] Synthesis of poly(Leu)40poly(GluOMe)80: 10 g of NCA-GluOMe are solubilized in 150 ml of NMP at 60° C. 5 ml of a solution of 0.91 g of benzylamine in 50 ml of NMP are added all at once to the monomer. After 1 h, 14.1 g of NCA-Leu, previously solubilized in 20 ml of NMP, are added. Polymerization continues for a further 3-4 h.
[0185] Sampling of the reaction medium affords the following characterizations: Yield: 90%. Composition by 1H NMR (TFA-d): 66 mol % of GluOMe. Reduced viscosity (0.5% of TFA at 25° C.): 0.4 dl / g. Molecular weight by GPC: 20,000 g / mol.
example 2
Aminolysis of Poly(Leucine / Methyl Glutamate) Block Copolymer with Hydroxyethylamine
[0186] 10 g of hydroxyethylamine are added all at once to the NMP solution of polymer prepared according to Example 1. The medium is heated to 80° C. and kept at this temperature for 2 days. 150 ml of water are then added to the polymer solution, which is then purified by a dialysis step to ensure removal of the excess amine and the NMP. Finally, the particles are isolated by lyophilization.
[0187] Yield: quantitative. 1H NMR (TFA-d): 8% of residual methoxy (3.5 ppm); 92% of hydroxyethylglutamine. Composition by 1H NMR (TFA-d): 34% of Leu. Particle size (light scattering, QLS mode): 100 nm.
example 3
Demonstration of Nanoparticles by Light Scattering (LS) and Transmission Electron Microscopy (TEM)
[0188] 10 mg of particles of polymer 1 are suspended in 10 ml of water or an aqueous solution of salt. This solution is then introduced into a Coulter granulometer (or laser diffractometer). The results of particle size analysis of the different products tested are shown in Table 1 below.
TABLE 1Measurement of the size of the CPExamplePolymerSize (nm)2poly[(Leu)0.66]-poly[(Gln-N-100hydroxyethyl)0.37]x block copolymer
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com